Relation between Mid-Regional Pro-Adrenomedullin in Patients with Chronic Heart Failure and the Dose of Diuretics in 2-Year Follow-Up—Data from FAR NHL Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Methods
2.3. Laboratory Data
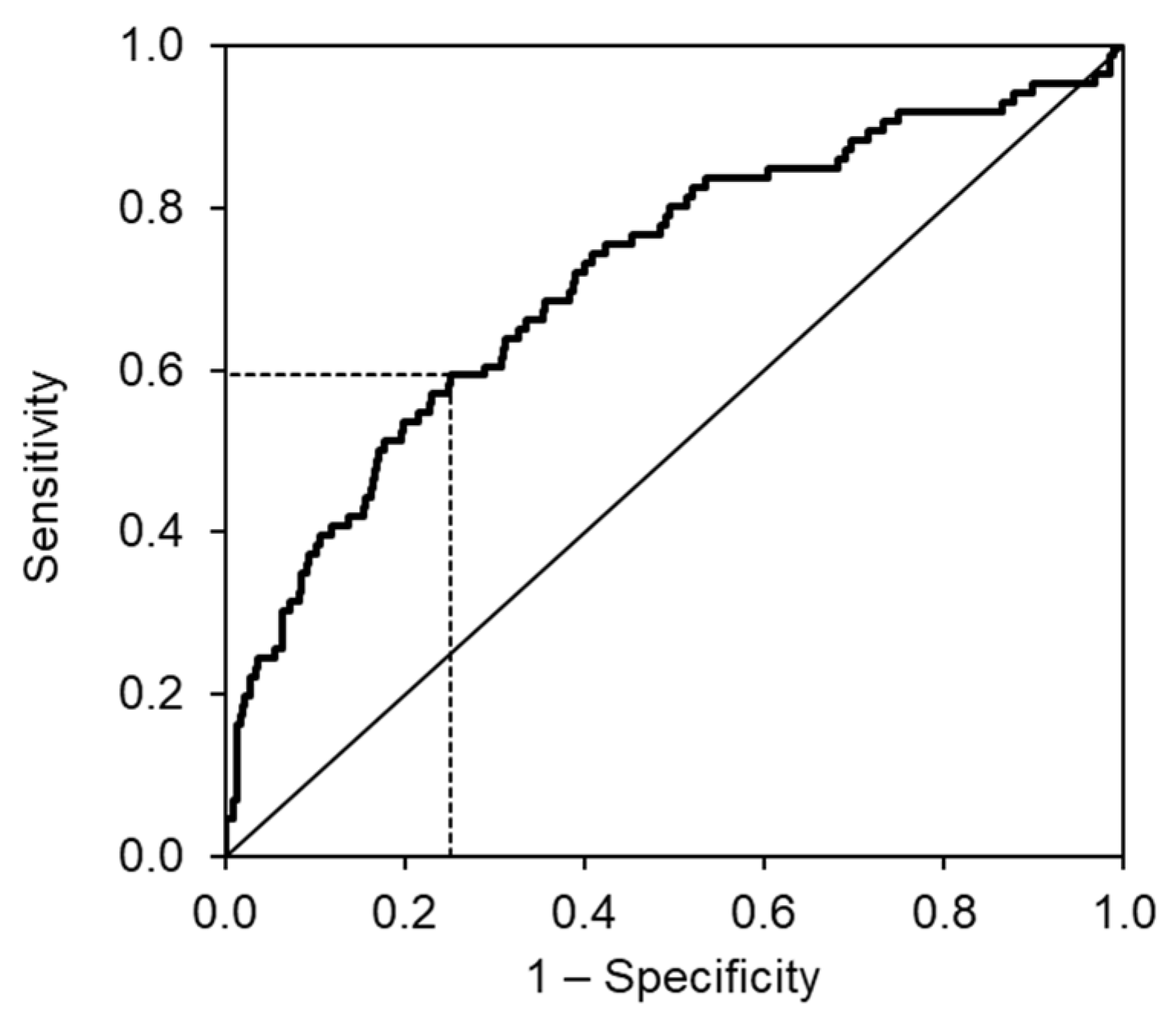
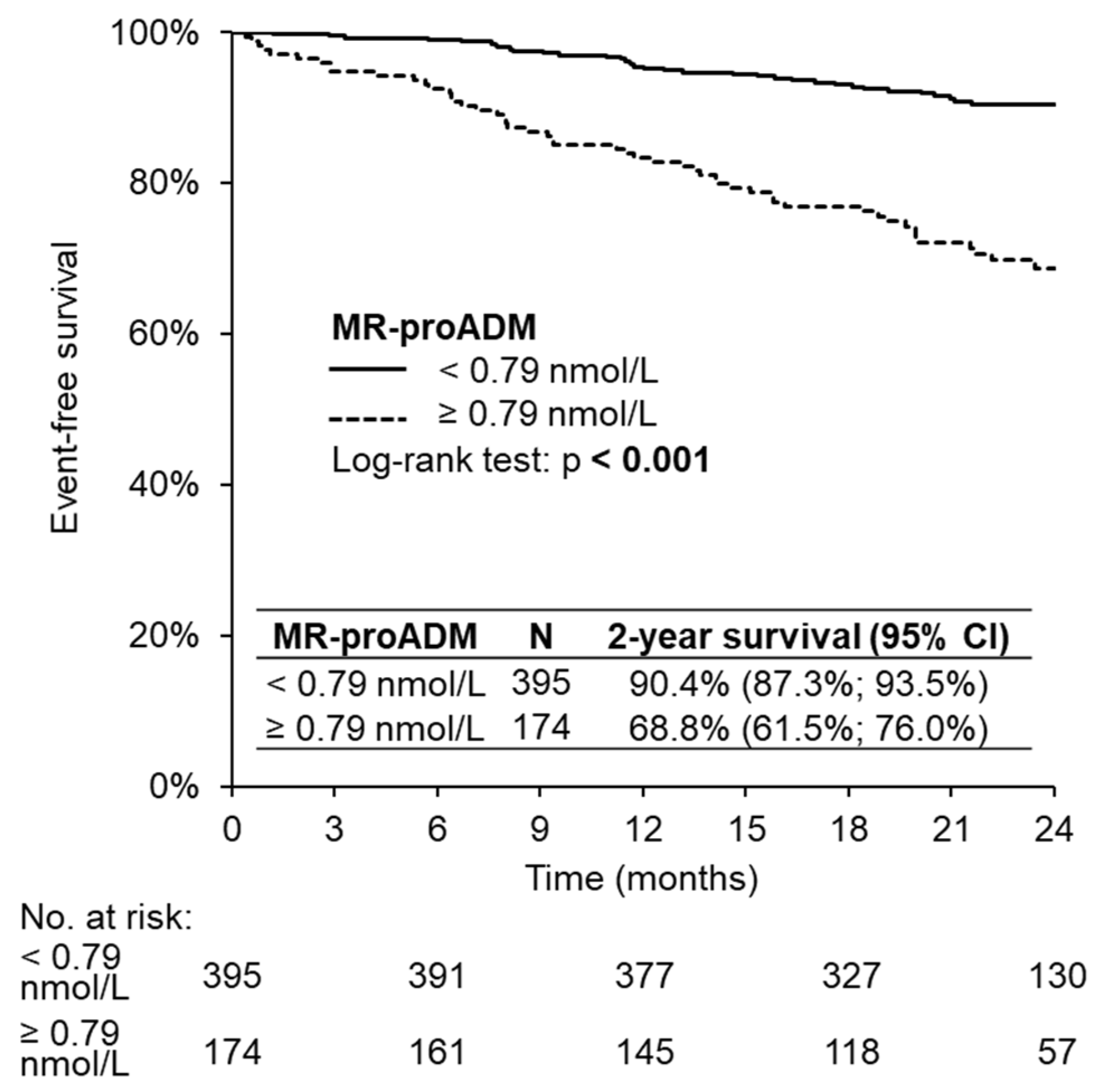
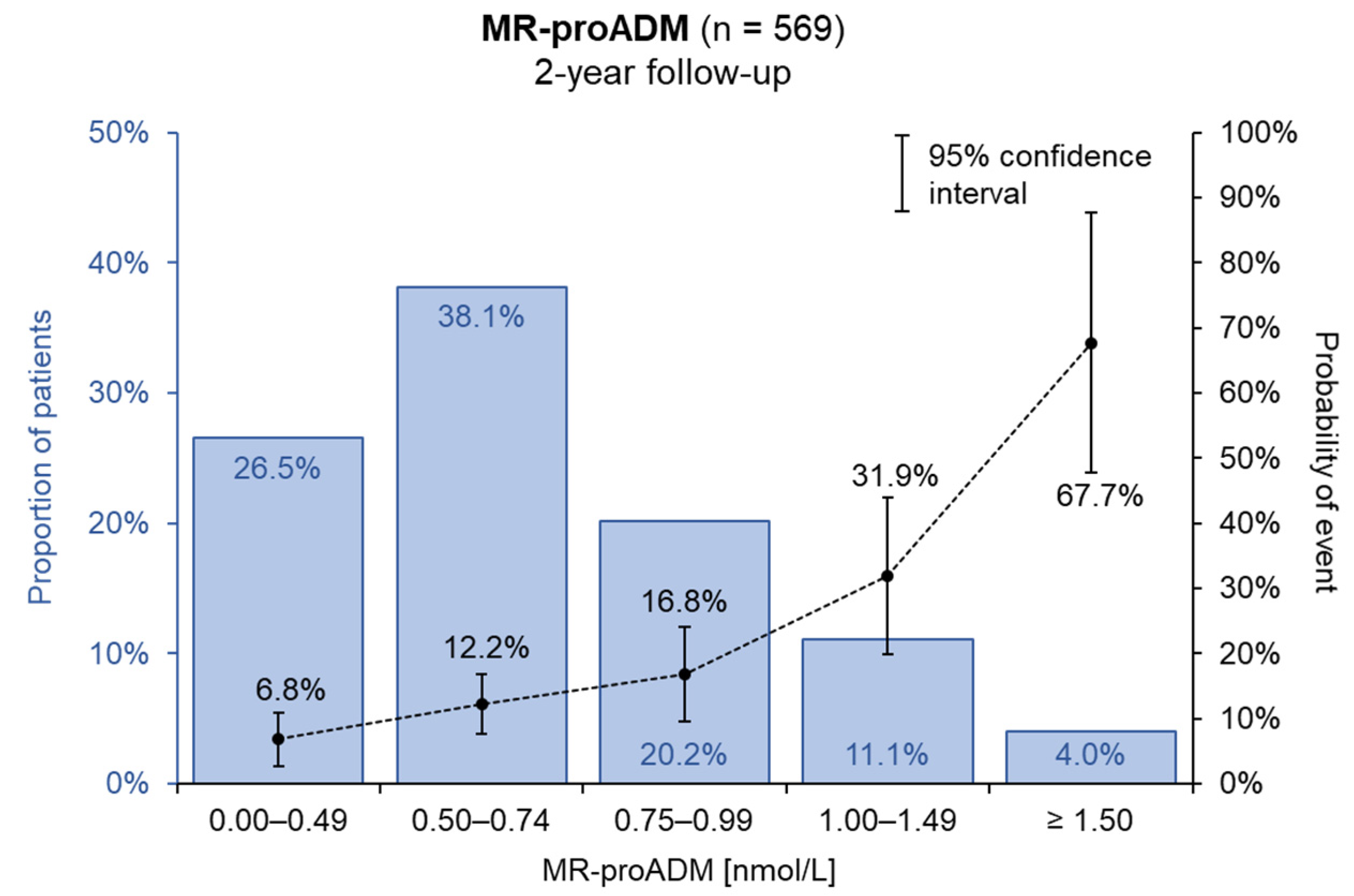
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Maisel, A.S.; Duran, J.M.; Wettersten, N. Natriuretic Peptides in Heart Failure: Atrial and B-type Natriuretic Peptides. Heart Fail. Clin. 2018, 14, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Spinar, J.; Spinarova, L.; Malek, F.; Ludka, O.; Krejci, J.; Ostadal, P.; Vondrakova, D.; Labr, K.; Spinarova, M.; Pavkova Goldbergova, M.; et al. Prognostic value of NT-proBNP added to clinical parameters to predict two-year prognosis of chronic heart failure patients with mid-range and reduced ejection fraction—A report from FAR NHL prospective registry. PLoS ONE 2019, 14, e0214363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piek, A.; Du, W.; de Boer, R.A.; Silljé, H.H.W. Novel heart failure biomarkers: Why do we fail to exploit their potential? Crit. Rev. Clin. Lab. Sci. 2018, 55, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 2000, 21, 138–167. [Google Scholar] [CrossRef]
- Hirano, S.; Imamura, T.; Matsuo, T.; Ishiyama, Y.; Kato, J.; Kitamura, K.; Koiwaya, Y.; Eto, T. Differential responses of circulating and tissue adrenomedullin and gene expression to volume overload. J. Card. Fail. 2000, 6, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.J.; Lainchbury, J.G.; Nicholls, M.G.; Rademaker, M.T.; Richards, A.M.; Troughton, R.W. Adrenomedullin and the renin-angiotensin-aldosterone system. Regul. Pept. 2003, 112, 41–49. [Google Scholar] [CrossRef]
- Jougasaki, M.; Wei, C.M.; McKinley, L.J.; Burnett, J.C. Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation 1995, 92, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.; Mueller, C.; Nowak, R.M.; Peacock, W.F.; Ponikowski, P.; Mockel, M.; Hogan, C.; Wu, A.H.B.; Richards, M.; Clopton, P.; et al. Midregion Prohormone Adrenomedullin and Prognosis in Patients Presenting With Acute Dyspnea: Results from the BACH (Biomarkers in Acute Heart Failure) Trial. J. Am. Coll. Cardiol. 2011, 58, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- Špinarová, L.; Špinarová, M.; Goldbergova-Pavkova, M. Prognostic Impact of Copeptin and Mid-Regional Pro-Adrenomedullin in Chronic Heart Failure with Regard to Comorbidities. J. Cardiovasc. Dis. Diagn. 2018, 6, 326. [Google Scholar] [CrossRef]
- Haehling, S. von Filippatos, G.S.; Papassotiriou, J.; Cicoira, M.; Jankowska, E.A.; Doehner, W.; Rozentryt, P.; Vassanelli, C.; Struck, J.; Banasiak, W.; et al. Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur. J. Heart Fail. 2010, 12, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation 2005, 112, e154–e235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinar, J.; Jarkovsky, J.; Spinarova, L.; Mebazaa, A.; Gayat, E.; Vitovec, J.; Linhart, A.; Widimsky, P.; Miklik, R.; Zeman, K.; et al. AHEAD score—Long-term risk classification in acute heart failure. Int. J. Cardiol. 2016, 202, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Swedberg, K.; Follath, F.; Komajda, M.; Cohen-Solal, A.; Aguilar, J.C.; Dietz, R.; Gavazzi, A.; Hobbs, R.; Korewicki, J.; et al. The EuroHeart Failure survey programme—A survey on the quality of care among patients with heart failure in Europe. Part 1: Patient characteristics and diagnosis. Eur. Heart J. 2003, 24, 442–463. [Google Scholar] [CrossRef] [Green Version]
- Gheorghiade, M.; Filippatos, G.; De Luca, L.; Burnett, J. Congestion in acute heart failure syndromes: An essential target of evaluation and treatment. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.N.L.; Arrigo, M.; Placido, R.; Akiyama, E.; Girerd, N.; Zannad, F.; Manivet, P.; Rossignol, P.; Badoz, M.; Sadoune, M.; et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur. J. Heart Fail. 2018, 20, 738–747. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Gracia, J.; Demissei, B.G.; ter Maaten, J.M.; Cleland, J.G.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 2018, 258, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Pérez Calvo, J.I.; Rubio Gracia, J.; Josa Laorden, C.; Morales Rull, J.L. La congestión residual y la intuición clínica en la insuficiencia cardiaca descompensada. Rev. Clínica Española 2019, 219, 327–331. [Google Scholar] [CrossRef] [PubMed]
- ter Maaten, J.M.; Valente, M.A.E.; Damman, K.; Hillege, H.L.; Navis, G.; Voors, A.A. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat. Rev. Cardiol. 2015, 12, 184–192. [Google Scholar] [CrossRef]
- Pham, D.; Grodin, J.L. Dilemmas in the Dosing of Heart Failure Drugs: Titrating Diuretics in Chronic Heart Failure. Card. Fail. Rev. 2017, 3, 108–112. [Google Scholar] [CrossRef]
- Weber, J.; Sachse, J.; Bergmann, S.; Sparwaßer, A.; Struck, J.; Bergmann, A. Sandwich Immunoassay for Bioactive Plasma Adrenomedullin. J. Appl. Lab. Med. 2017, 2, 222–233. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Description * |
|---|---|
| Basic characteristics | |
| Sex—male | 459 (80.7%) |
| Age (years) | 65 ± 12 |
| BMI | 29 ± 5 |
| SBP (mmHg) | 127 ± 16 |
| DBP (mmHg) | 80 ± 10 |
| Heart rate (min−1) | 74 ± 13 |
| LV EF (%) | 31 ± 9 |
| Ischemic aetiology of HF | 303 (53.3%) |
| Hypertension | 365 (64.1%) |
| Diabetes mellitus | 229 (40.2%) |
| COPD | 82 (14.4%) |
| Chronic lower extremity ischemia | 56 (9.8%) |
| Smoking | |
| Non-smoker | 326 (57.3%) |
| Smoker | 56 (9.8%) |
| Ex-smoker | 187 (32.9%) |
| NYHA classification | |
| 1 | 78 (13.7%) |
| 2 | 377 (66.3%) |
| 3 + 4 | 114 (20.0%) |
| Laboratory results | |
| MR-proADM (nmol/L) | 0.64 (0.49; 0.84) |
| NT-proBNP (ng/L) | 662 (216; 1 838) |
| Haemoglobin (g/L) | 144 (133; 153) |
| Natrium (mmol/L) | 141 (139; 143) |
| Urea (mmol/L) | 6.1 (4.9; 8.2) |
| Uric acid (µmol/L) | 399 (339; 467) |
| Creatinine (μmol/L) | 96 (83; 116) |
| eGFR (mL/min/1.73 m2) | 69 (51; 84) |
| Medication | |
| ACEI/ARB | 503 (88.4%) |
| Beta-blockers | 530 (93.1%) |
| Furosemide | 447 (78.6%) |
| Hydrochlorothiazide | 73 (12.8%) |
| Spironolactone/eplerenone | 365 (64.1%) |
| Parameter | Category | N | Level of MR-proADM (nmol/L) | p | ||
|---|---|---|---|---|---|---|
| Mean (±SD) | Median (25th Percentile; 75th Percentile) | OR (95% CI) | ||||
| Sex | Men | 459 | 0.73 (±0.46) | 0.64 (0.48; 0.83) | 1.16 (0.64–2.11) | NS |
| Women | 110 | 0.80 (±0.58) | 0.66 (0.53; 0.89) | |||
| Age | <65 years | 266 | 0.58 (±0.25) | 0.53 (0.42; 0.68) | 1.68 (1.05–2.70) | <0.001 |
| ≥65 years | 303 | 0.89 (±0.58) | 0.75 (0.60; 0.98) | |||
| BMI | <30 | 340 | 0.72 (±0.46) | 0.63 (0.49; 0.82) | 1.07 (0.67–1.71) | NS |
| ≥30 | 225 | 0.78 (±0.52) | 0.67 (0.50; 0.86) | |||
| LV EF | <40 | 441 | 0.76 (±0.53) | 0.66 (0.49; 0.85) | 0.51 (0.27–0.98) | NS |
| ≥40 | 128 | 0.67 (±0.28) | 0.60 (0.49; 0.78) | |||
| NYHA | 1 | 78 | 0.54 (±0.22) | 0.49 (0.40; 0.67) | <0.001 | |
| 2 | 377 | 0.77 (±0.50) | 0.66 (0.53; 0.87) | 2.70 (0.94–7.72) | ||
| 3 + 4 | 114 | 0.80 (±0.54) | 0.68 (0.51; 0.97) | 7.86 (2.66–23.23) | ||
| Ischemic aetiology | No | 266 | 0.70 (±0.44) | 0.60 (0.47; 0.80) | 1.07 (0.67–1.69) | 0.013 |
| Yes | 303 | 0.78 (±0.51) | 0.68 (0.54; 0.89) | |||
| Hypertension | No | 204 | 0.64 (±0.30) | 0.61 (0.45; 0.77) | 1.44 (0.87–2.37) | <0.001 |
| Yes | 365 | 0.80 (±0.55) | 0.67 (0.53; 0.91) | |||
| Atrial fibrillation | No | 373 | 0.65 (±0.32) | 0.58 (0.47; 0.76) | 1.02 (0.63–1.66) | <0.001 |
| Yes | 196 | 0.92 (±0.67) | 0.76 (0.62; 1.02) | |||
| Diabetes mellitus | No | 340 | 0.66 (±0.37) | 0.60 (0.46; 0.78) | 1.60 (1.01–2.53) | <0.001 |
| Yes | 229 | 0.86 (±0.60) | 0.71 (0.57; 0.98) | |||
| Swollen lower limb | No | 477 | 0.69 (±0.35) | 0.62 (0.48; 0.80) | 2.03 (1.17–3.50) | <0.001 |
| Yes | 92 | 1.01 (±0.86) | 0.78 (0.63; 1.08) | |||
| eGFR [mL/min/1.73 m2] | <60 | 200 | 1.03 (±0.67) | 0.84 (0.68; 1.17) | 0.54 (0.34–0.86) | <0.001 |
| ≥60 | 369 | 0.59 (±0.22) | 0.55 (0.45; 0.69) | |||
| Parameter | Category | OR (95% CI) | p-Value |
|---|---|---|---|
| Dose of furosemide [mg/day] | 1–39 (ref. 0) | 0.79 (0.27–2.26) | 0.656 |
| 40–79 (ref. 0) | 1.58 (0.68–3.67) | 0.291 | |
| 80–119 (ref. 0) | 2.76 (1.04–7.32) | 0.042 | |
| ≥120 (ref. 0) | 6.03 (2.46–14.79) | <0.001 | |
| MR-proADM | Increase of 0.1 nmol/L | 1.14 (1.07–1.21) | <0.001 |
| MR-proADM [nmol/L] | ||||||
|---|---|---|---|---|---|---|
| 0.00–0.49 | 0.50–0.74 | 0.75–0.99 | 1.00–1.49 | ≥1.50 | ||
| Dose of furosemide [mg/day] | 0 | 4.8% | 7.1% | 9.9% | 18.6% | low N |
| 1–39 | 3.5% | 5.1% | 7.2% | 13.9% | low N | |
| 40–79 | 6.9% | 10.1% | 13.9% | 25.1% | 46.0% | |
| 80–119 | 10.8% | 15.5% | 20.8% | 35.4% | 58.2% | |
| 120+ | 21.7% | 29.4% | 37.4% | 55.4% | 76.0% | |
| Parameter | Category | N | Level of MR-proADM (nmol/L) | p | |
|---|---|---|---|---|---|
| Mean Level of (±SD) | Median (25th Percentile; 75th Percentile) | ||||
| Dose of furosemide | 0 mg/day | 122 | 0.62 (±0.55) | 0.55 (0.46; 0.67) | <0.001 |
| 1–39 mg/day | 113 | 0.67 (±0.30) | 0.60 (0.44; 0.82) | ||
| 40–79 mg/day | 202 | 0.72 (±0.34) | 0.67 (0.51; 0.81) | ||
| 80–119 mg/day | 58 | 0.85 (±0.40) | 0.74 (0.58; 0.99) | ||
| ≥120 mg/day | 74 | 1.07 (±0.76) | 0.82 (0.61; 1.23) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Špinarová, M.; Špinar, J.; Špinarová, L.; Krejčí, J.; Goldbergová-Pávková, M.; Pařenica, J.; Ludka, O.; Málek, F.; Ošťádal, P.; Benešová, K.; et al. Relation between Mid-Regional Pro-Adrenomedullin in Patients with Chronic Heart Failure and the Dose of Diuretics in 2-Year Follow-Up—Data from FAR NHL Registry. Medicina 2022, 58, 1477. https://doi.org/10.3390/medicina58101477
Špinarová M, Špinar J, Špinarová L, Krejčí J, Goldbergová-Pávková M, Pařenica J, Ludka O, Málek F, Ošťádal P, Benešová K, et al. Relation between Mid-Regional Pro-Adrenomedullin in Patients with Chronic Heart Failure and the Dose of Diuretics in 2-Year Follow-Up—Data from FAR NHL Registry. Medicina. 2022; 58(10):1477. https://doi.org/10.3390/medicina58101477
Chicago/Turabian StyleŠpinarová, Monika, Jindřich Špinar, Lenka Špinarová, Jan Krejčí, Monika Goldbergová-Pávková, Jiří Pařenica, Ondřej Ludka, Filip Málek, Petr Ošťádal, Klára Benešová, and et al. 2022. "Relation between Mid-Regional Pro-Adrenomedullin in Patients with Chronic Heart Failure and the Dose of Diuretics in 2-Year Follow-Up—Data from FAR NHL Registry" Medicina 58, no. 10: 1477. https://doi.org/10.3390/medicina58101477
APA StyleŠpinarová, M., Špinar, J., Špinarová, L., Krejčí, J., Goldbergová-Pávková, M., Pařenica, J., Ludka, O., Málek, F., Ošťádal, P., Benešová, K., Jarkovský, J., & Lábr, K. (2022). Relation between Mid-Regional Pro-Adrenomedullin in Patients with Chronic Heart Failure and the Dose of Diuretics in 2-Year Follow-Up—Data from FAR NHL Registry. Medicina, 58(10), 1477. https://doi.org/10.3390/medicina58101477







