The Effect and Safety of Rapid and Gradual Urinary Decompression in Urine Retention: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol
2.2. Databases and Search Strategy
2.3. Data Extraction and Assessment of Methodological Quality
2.4. Data Collection, Data Processing, and Primary Data Analysis
2.5. Grading of the Certainty of Evidence
3. Results
3.1. Search Results
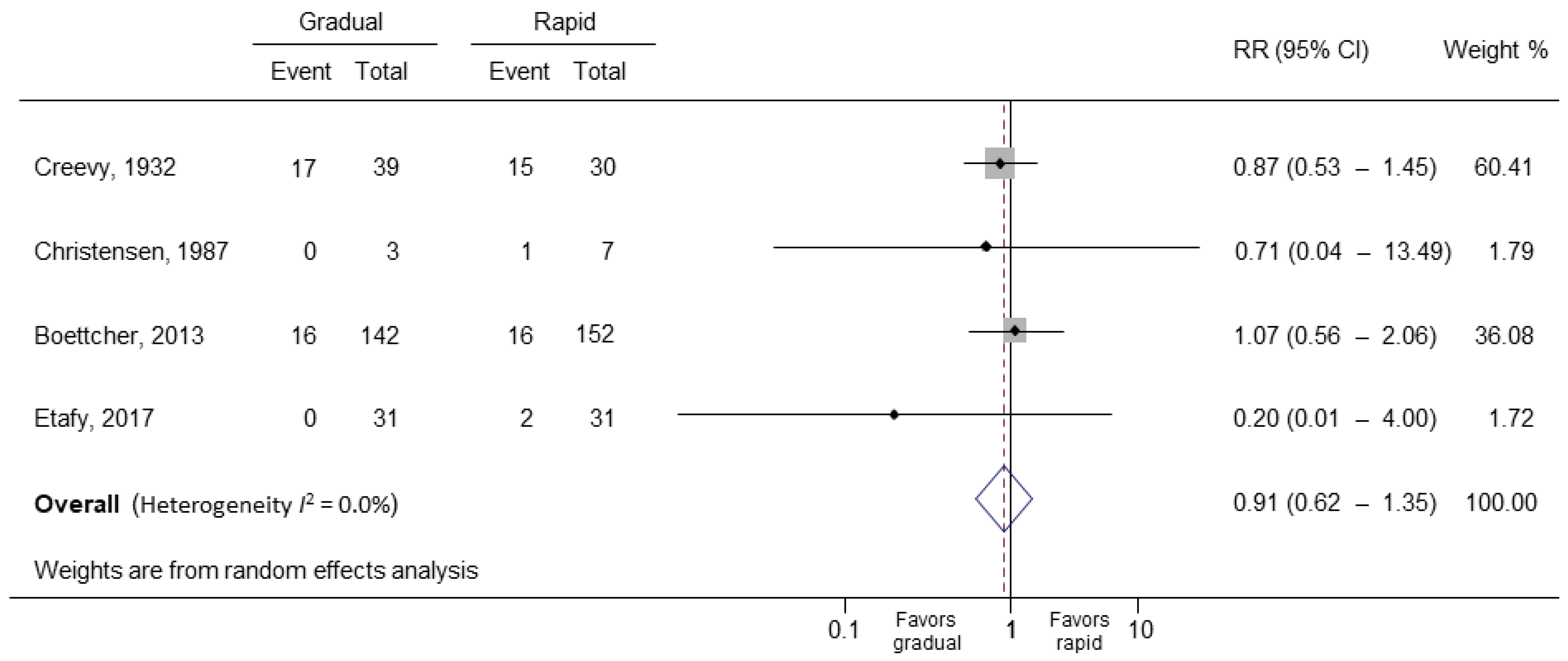
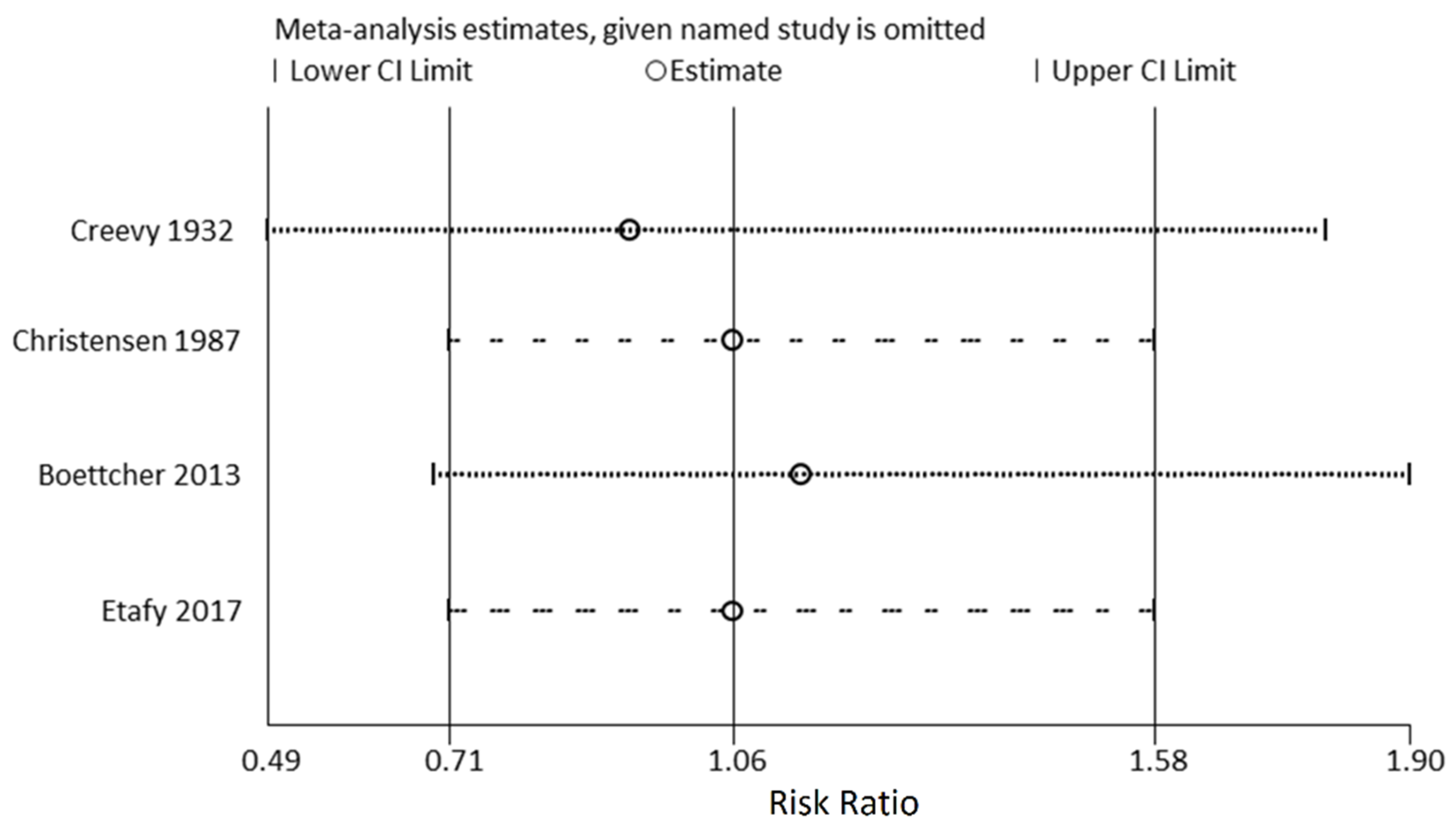
3.2. Main Outcomes
3.3. CoE Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fong, Y.K.; Milani, S.; Djavan, B. Natural history and clinical predictors of clinical progression in benign prostatic hyperplasia. Curr. Opin. Urol. 2005, 15, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.A.; Wein, A.J.; Staskin, D.R.; Roehrborn, C.G.; Steers, W.D. Urinary retention and post-void residual urine in men: Separating truth from tradition. J. Urol. 2008, 180, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Choong, S.; Emberton, M. Acute urinary retention. BJU Int. 2000, 85, 186–201. [Google Scholar] [CrossRef]
- Manjunath, A.S.; Hofer, M.D. Urologic Emergencies. Med. Clin. N. Am. 2018, 102, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Maldonado, M.; Kumjian, D.A. Acute renal failure due to urinary tract obstruction. Med. Clin. N. Am. 1990, 74, 919–932. [Google Scholar] [CrossRef]
- Nyman, M.A.; Schwenk, N.M.; Silverstein, M.D. Management of urinary retention: Rapid versus gradual decompression and risk of complications. Mayo Clin. Proc. 1997, 72, 951–956. [Google Scholar] [CrossRef]
- Gray, M. Urinary retention. Management in the acute care setting. Part. 2. Am. J. Nurs. 2000, 100, 36–43, quiz 44. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Djulbegovic, B.; Guyatt, G.H. Progress in evidence-based medicine: A quarter century on. Lancet 2017, 390, 415–423. [Google Scholar] [CrossRef]
- Creevy, C.D. Sudden decompression of the chronically distended urinary bladder: A clinical and pathologic study. Arch. Surg. 1932, 25, 356–385. [Google Scholar] [CrossRef]
- Seifert, E. Die Blasenblutung ex vacuo. Zentralbl. Chir. 1940, 67, 550–554. [Google Scholar]
- Lapides, J.; Lovegrove, R.H. Urinary vesicovascular reflex. J. Urol. 1965, 94, 397–401. [Google Scholar] [CrossRef]
- Paquin, J.M.; Paquin, J.M.; Perreault, J.P. Traitement de 1aphase aigue de la retention urinaire. J. Urol. 1981, 87, 37–38. [Google Scholar]
- Taylor, D.E. Viscero-vascular reflexes and the surgical patient. J. R. Coll. Surg. Edinb. 1966, 12, 61–67. [Google Scholar]
- Glahn, B.E.; Plucnar, B.J. Quick complete emptying of the bladder in 300 cases of urinary retention. The occurrence of haematuria. Dan. Med. Bull. 1984, 31, 68–70. [Google Scholar]
- Boettcher, S.; Brandt, A.S.; Roth, S.; Mathers, M.J.; Lazica, D.A. Urinary retention: Benefit of gradual bladder decompression—Myth or truth? A randomized controlled trial. Urol. Int. 2013, 91, 140–144. [Google Scholar] [CrossRef]
- Christensen, J.; Ostri, P.; Frimodt-Møller, C.; Juul, C. Intravesical pressure changes during bladder drainage in patients with acute urinary retention. Urol. Int. 1987, 42, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Etafy, M.H.; Saleh, F.H.; Ortiz-Vanderdys, C.; Hamada, A.; Refaat, A.M.; Aal, M.A.; Deif, H.; Gawish, M.; Abdellatif, A.H.; Gadalla, K. Rapid versus gradual bladder decompression in acute urinary retention. Urol. Ann. 2017, 9, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, A.; Abubakar, A. Pathophysiology and management of urinary retention in men. Arch. Int. Surg. 2012, 2, 63–69. [Google Scholar] [CrossRef]
- Mitchell, J.P. Management of chronic urinary retention. Br. Med. J. 1984, 289, 515–516. [Google Scholar] [CrossRef]
- Ahmed, M.; Abubakar, A.; Lawal, A.T.; Bello, A.; Maitama, H.Y.; Mbibu, H.N. Rapid and complete decompression of chronic urinary retention: A safe and effective practice. Trop. Doct. 2013, 43, 13–16. [Google Scholar] [CrossRef]
- Gould, F.; Cheng, C.Y.; Lapides, J. Comparison of rapid versus slow decompression of the distended urinary bladder. Investig. Urol. 1976, 14, 156–158. [Google Scholar]
- Tammela, T.; Autio-Harmainen, H.; Lukkarinen, O.; Sormunen, R. Effect of distension on function and nervous ultrastructure in the canine urinary bladder. Urol. Int. 1987, 42, 265–270. [Google Scholar] [CrossRef]
- Gabriel, C.; Suchard, J.R. Hematuria Following Rapid Bladder Decompression. Clin. Pract. Cases Emerg. Med. 2017, 1, 443–445. [Google Scholar] [CrossRef][Green Version]
- O’Conor, V.J. Observations on the blood pressure in cases of prostatic obstruction. Arch. Surg. 1920, 1, 359–367. [Google Scholar] [CrossRef][Green Version]
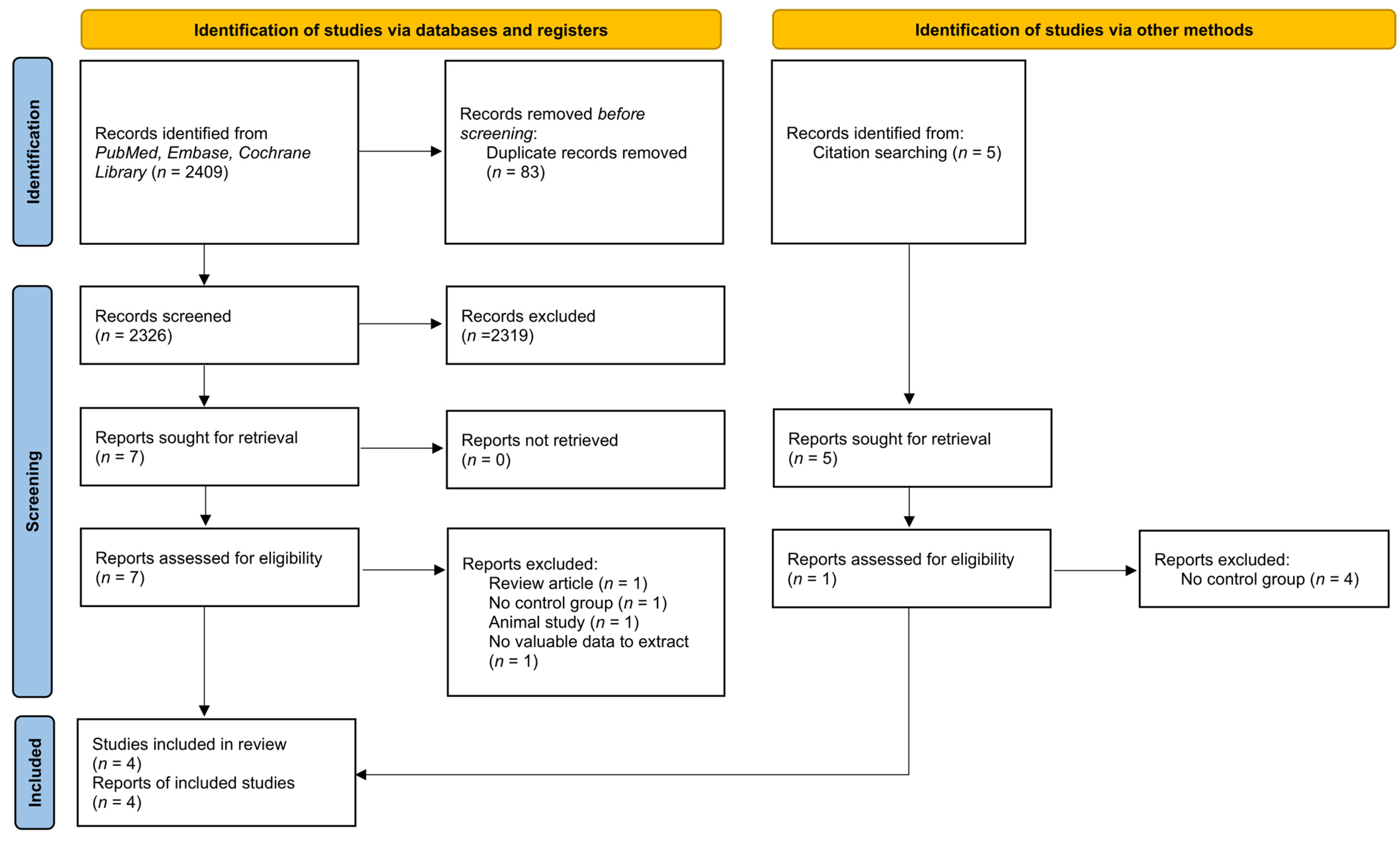

| PubMed | (“Urinary Retention” [All fields] OR “Bladder” [All fields] OR “Intravesical pressure” [All fields]) AND (Decompression* [All fields] OR Decompress* [all fields] OR drainage [all fields] OR Rapid [All fields] OR Gradual [All fields] OR Rate [All fields] OR “Urinary Catheterization” [All fields] OR “Urinary Catheters” [All fields]) AND (Hematuria [All fields] OR hypotension [All fields] OR diuresis [All fields]) |
| Embase | (“Urinary Retention” OR “Bladder” OR “Intravesical pressure”) AND (Decompression OR Decompress OR drainage OR “Urinary Catheterization” OR “Urinary Catheters”) AND (Hematuria OR bleeding OR hypotension OR Circulation OR diuresis) |
| Study | Year | Mean Age (Year) | Male (%) | Study Size | Acute or Chronic Urinary Retention | Catheter Size | Method of Decompression | |
|---|---|---|---|---|---|---|---|---|
| Rapid | Gradual | |||||||
| Creevy | 1932 | 67.2 | 100 * | 69 | Chronic | NA | Emptied promptly at a single session | Decompressed gradually in from one to five days |
| Christensen | 1987 | 74.5 † | 100 | 10 | Acute | PVC Foley 3-way catheter 20 Fr. | Complete continuous drainage | Fractionated drainage was performed in decrements of 100 cm3, allowing a steady state to elaborate between drainage periods |
| Boettcher | 2013 | 72.5 | 100 | 294 | Both | Chosen with regard to patient history | Drained completely by placing the drainage bag at a lower level than the bladder. | After each 200 mL of urine drained, the catheter was clamped for 5 min and then reopened until the bladder was completely empty |
| Etafy | 2017 | 63.8 | 100 * | 62 | Acute | Foley catheter, no mentioned size | Managed by rapid drainage of the bladder | The first 100 mL were immediately evacuated, then the rest was evacuated gradually in 2 h |
| Risk of Bias Domain | Boettcher, 2013 | Etafy, 2017 |
|---|---|---|
| Randomization process | Low risk | Some concerns |
| Deviations from intended interventions | Some concerns | Low risk |
| Missing outcome data | Low risk | Low risk |
| Measurement of the outcome | Low risk | Low risk |
| Selection of the reported result | Low risk | Some concerns |
| Overall risk of bias | Some concerns | Some concerns |
| Risk of Bias Domain | Creevy, 1932 | Christensen, 1987 |
|---|---|---|
| Bias due to confounding | Serious | Moderate |
| Bias in selection of participants into the study | Moderate | Low |
| Bias in classification of interventions | Serious | Low |
| Bias due to deviations from intended interventions | Serious | Moderate |
| Bias due to missing data | Serious | Low |
| Bias in measurement of outcomes | Moderate | Moderate |
| Bias in selection of the reported result | Moderate | Low |
| Overall bias | Serious | Moderate |
| Study | Year | Mean Age (Year) | Male (%) | Study Size | Events of Complication in Different Rate of Bladder Decompression n (%) | |
|---|---|---|---|---|---|---|
| Gradual | Rapid | |||||
| Hematuria | ||||||
| Creevy | 1932 | 67.2 | 100 | 69 | 17 (43.5) | 15 (50.0) |
| Christensen | 1987 | 74.5 † | 100 | 10 | 0 | 1 (14.3) |
| Boettcher | 2013 | 72.5 | 100 | 294 | 16 (11.3) | 16 (10.5) |
| Etafy | 2017 | 63.8 | 100 | 62 | 0 | 2 (6.5) |
| Seifert * | 1940 | NA | NA | 126 | NA | 3 (2) |
| Paquin * | 1981 | NA | NA | 50 | NA | 6 (12) |
| Glahn * | 1984 | 62 | NA | 300 | NA | 48 (16) |
| Circulation collapse | ||||||
| Creevy | 1932 | 67.2 | 100 | 69 | 0 | 0 |
| Lapides | 1965 | NA | NA | 40 | NA | 0 |
| Taylor * | 1966 | NA | NA | 18 | NA | 0 |
| Glahn * | 1984 | 62 | NA | 300 | NA | 0 |
| Christensen | 1987 | 74.5 † | 100 | 10 | 0 | 0 |
| Boettcher | 2013 | 72.5 | 100 | 294 | 0 | 0 |
| Etafy | 2017 | 63.8 | 100 | 62 | 0 | 0 |
| No of Trials (No of Patients) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Pooled RR (95% CI) | Overall Quality of Evidence |
|---|---|---|---|---|---|---|---|
| Haematuria | |||||||
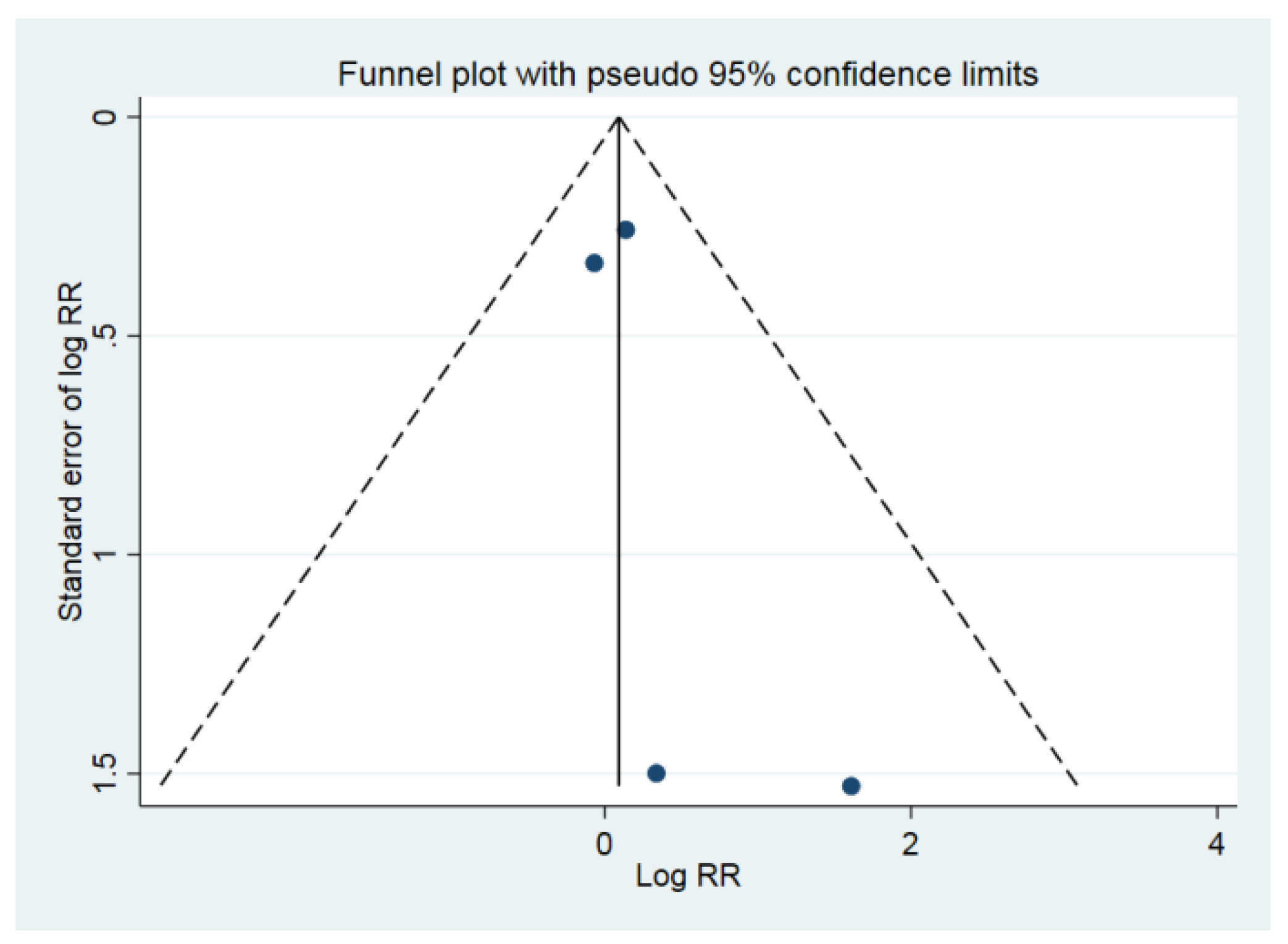
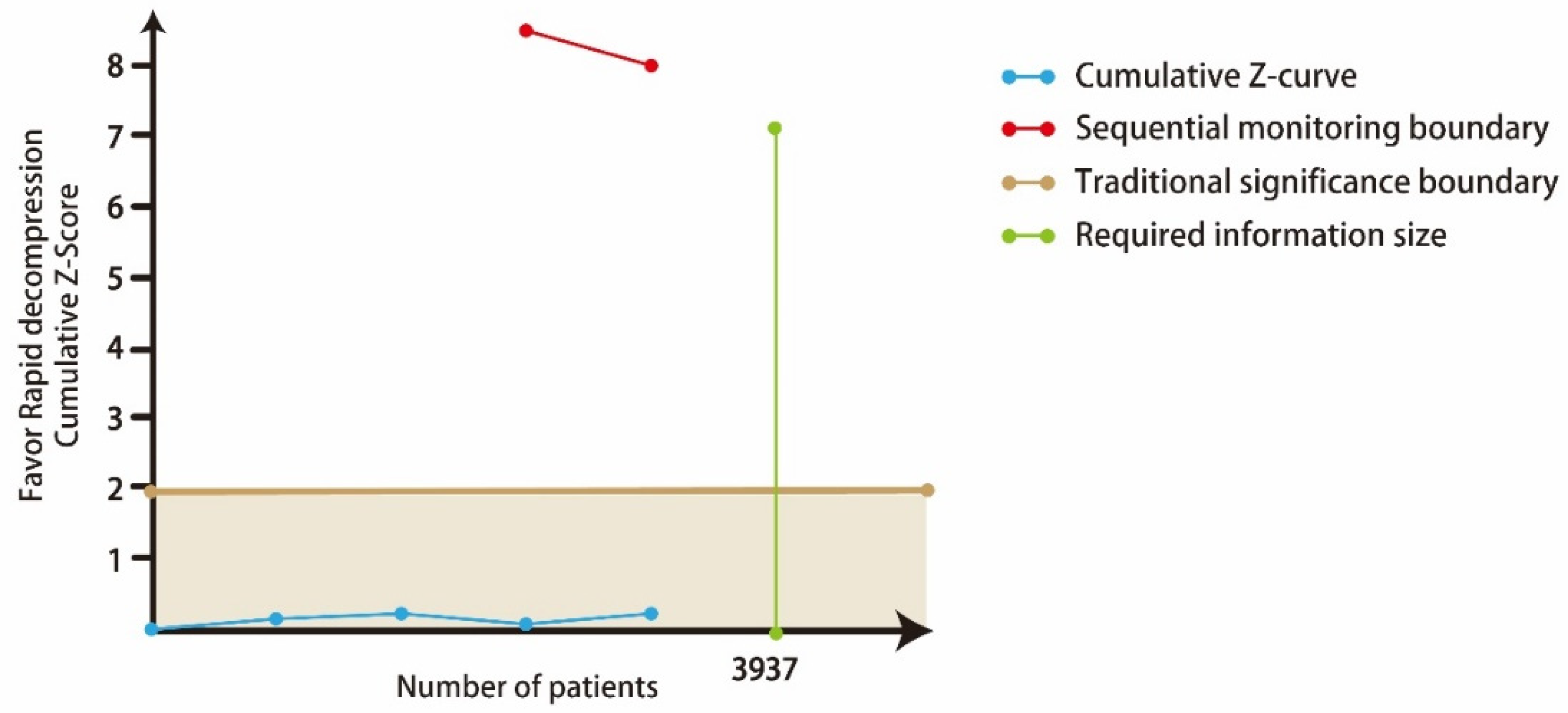
| 4 (435) | Very serious | Not downgraded I2 = 0.0% | Not downgraded | Downgraded | Not downgraded Begg’s test p = 0.497 | 0.91 (0.62–1.35) | ⊕⊖⊖⊖ VERY LOW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-Y.; Chang, J.-R.; Lee, Y.-K.; Lin, P.-C.; Tsai, T.-Y. The Effect and Safety of Rapid and Gradual Urinary Decompression in Urine Retention: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 1441. https://doi.org/10.3390/medicina58101441
Wu M-Y, Chang J-R, Lee Y-K, Lin P-C, Tsai T-Y. The Effect and Safety of Rapid and Gradual Urinary Decompression in Urine Retention: A Systematic Review and Meta-Analysis. Medicina. 2022; 58(10):1441. https://doi.org/10.3390/medicina58101441
Chicago/Turabian StyleWu, Meng-Yu, Jer-Ruey Chang, Yi-Kung Lee, Po-Chen Lin, and Tou-Yuan Tsai. 2022. "The Effect and Safety of Rapid and Gradual Urinary Decompression in Urine Retention: A Systematic Review and Meta-Analysis" Medicina 58, no. 10: 1441. https://doi.org/10.3390/medicina58101441
APA StyleWu, M.-Y., Chang, J.-R., Lee, Y.-K., Lin, P.-C., & Tsai, T.-Y. (2022). The Effect and Safety of Rapid and Gradual Urinary Decompression in Urine Retention: A Systematic Review and Meta-Analysis. Medicina, 58(10), 1441. https://doi.org/10.3390/medicina58101441







