Surveillance versus Adjuvant Treatment with Chemotherapy or Radiotherapy for Stage I Seminoma: A Systematic Review and Meta-Analysis According to EAU COVID-19 Recommendations
Abstract
1. Introduction
2. Materials and Methods
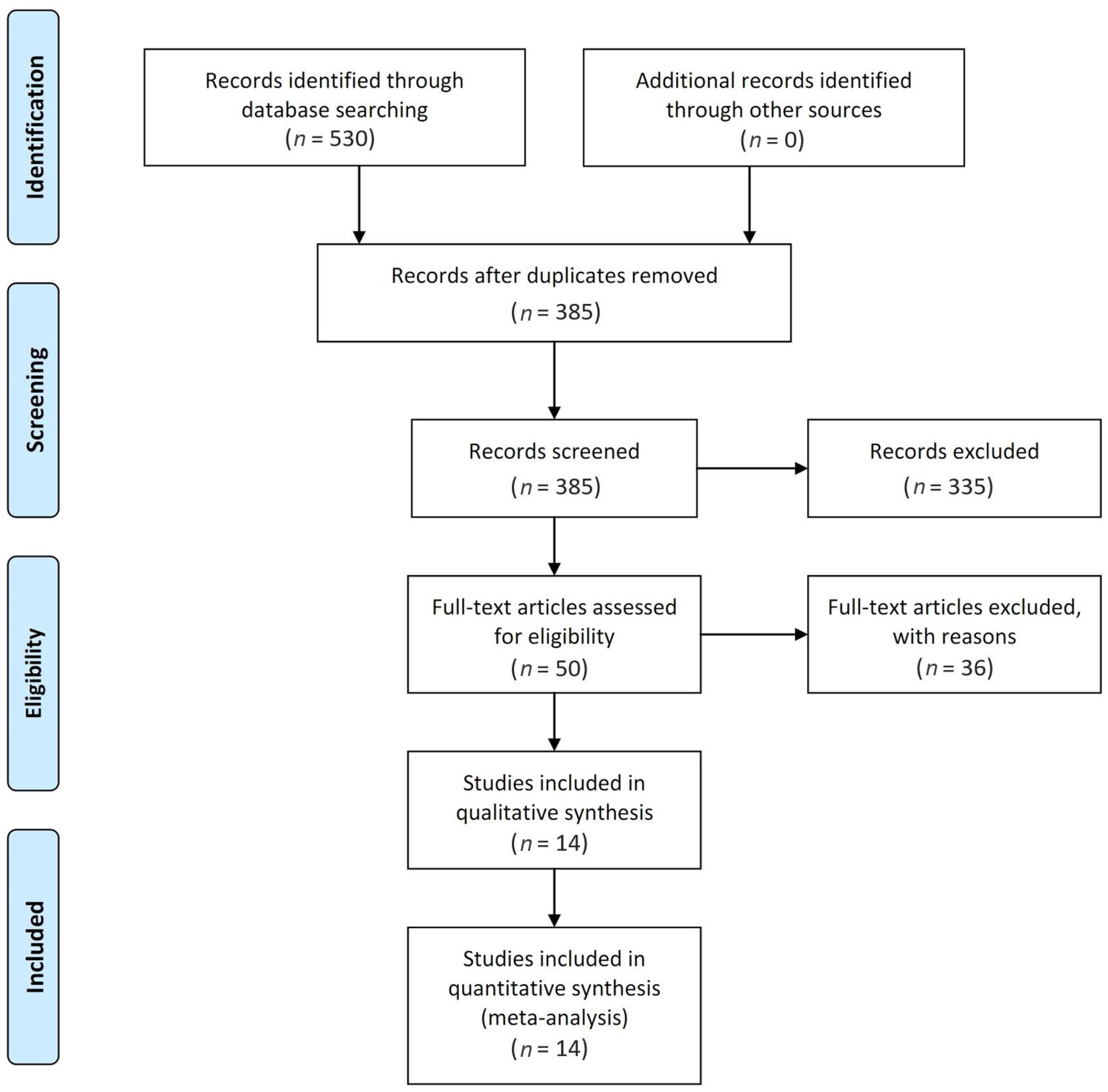
2.1. Literature Search and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
2.5. Ethics Statement
3. Results
3.1. Study Characteristics
3.2. Quality Assessment
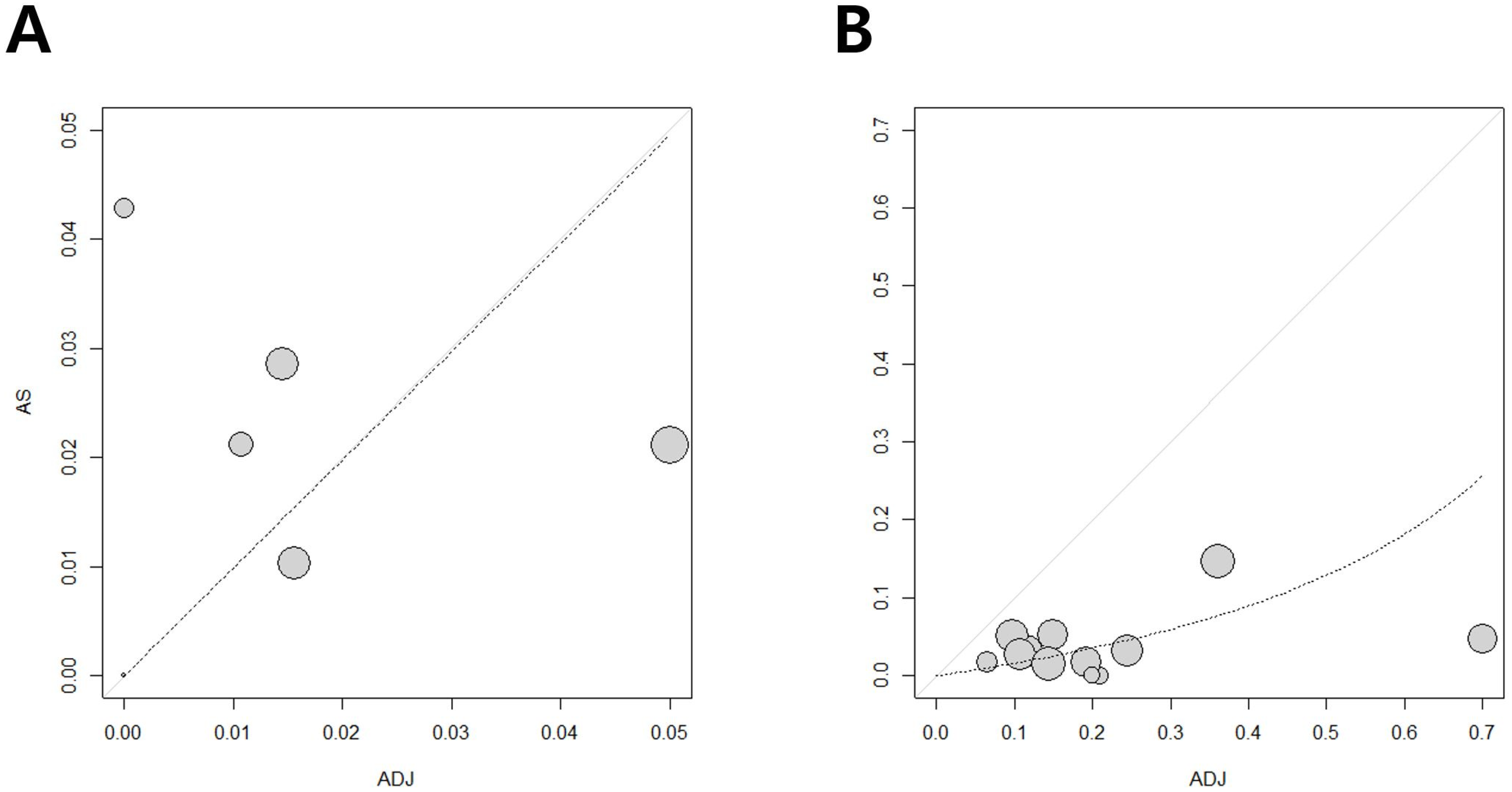
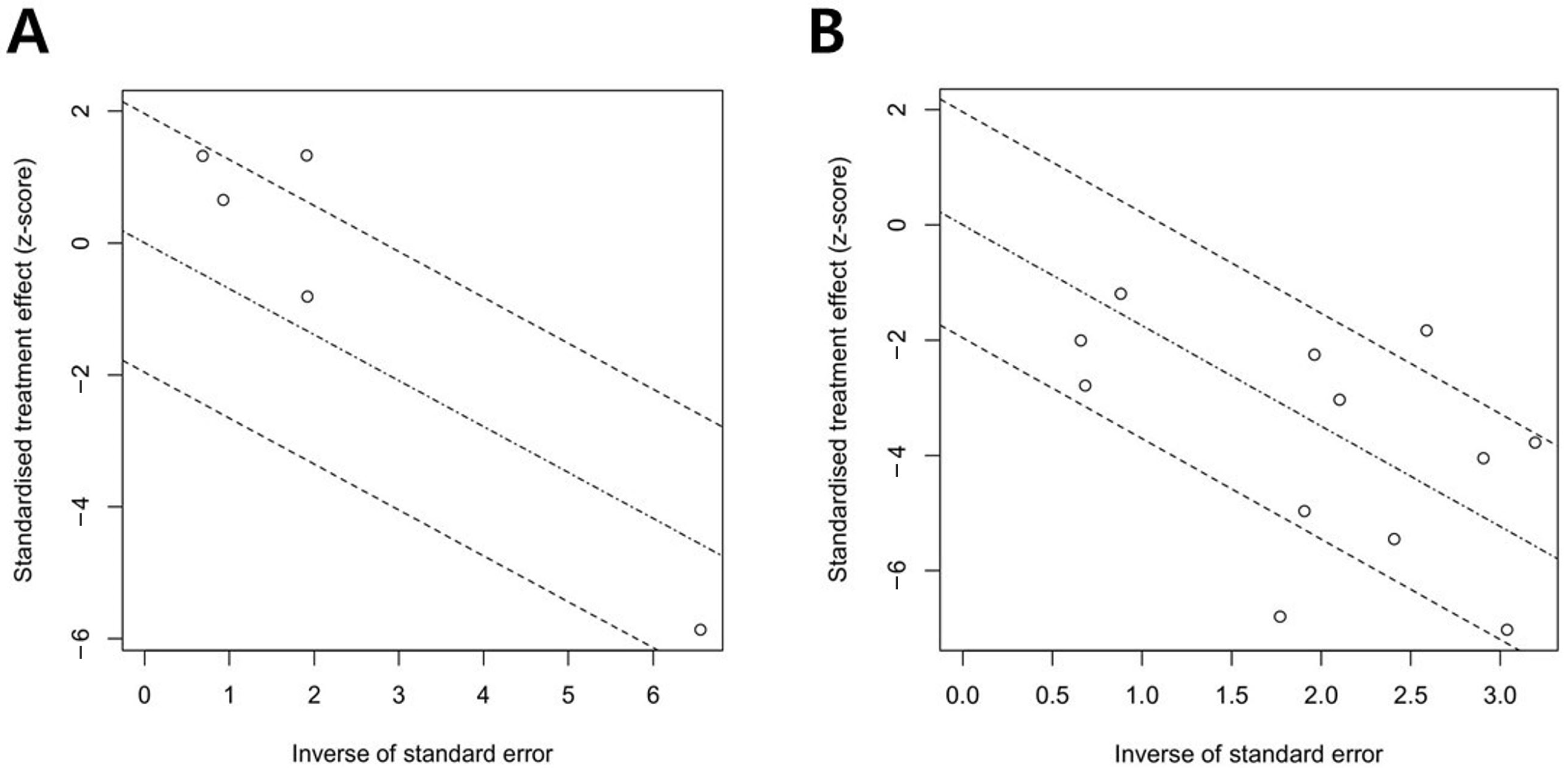
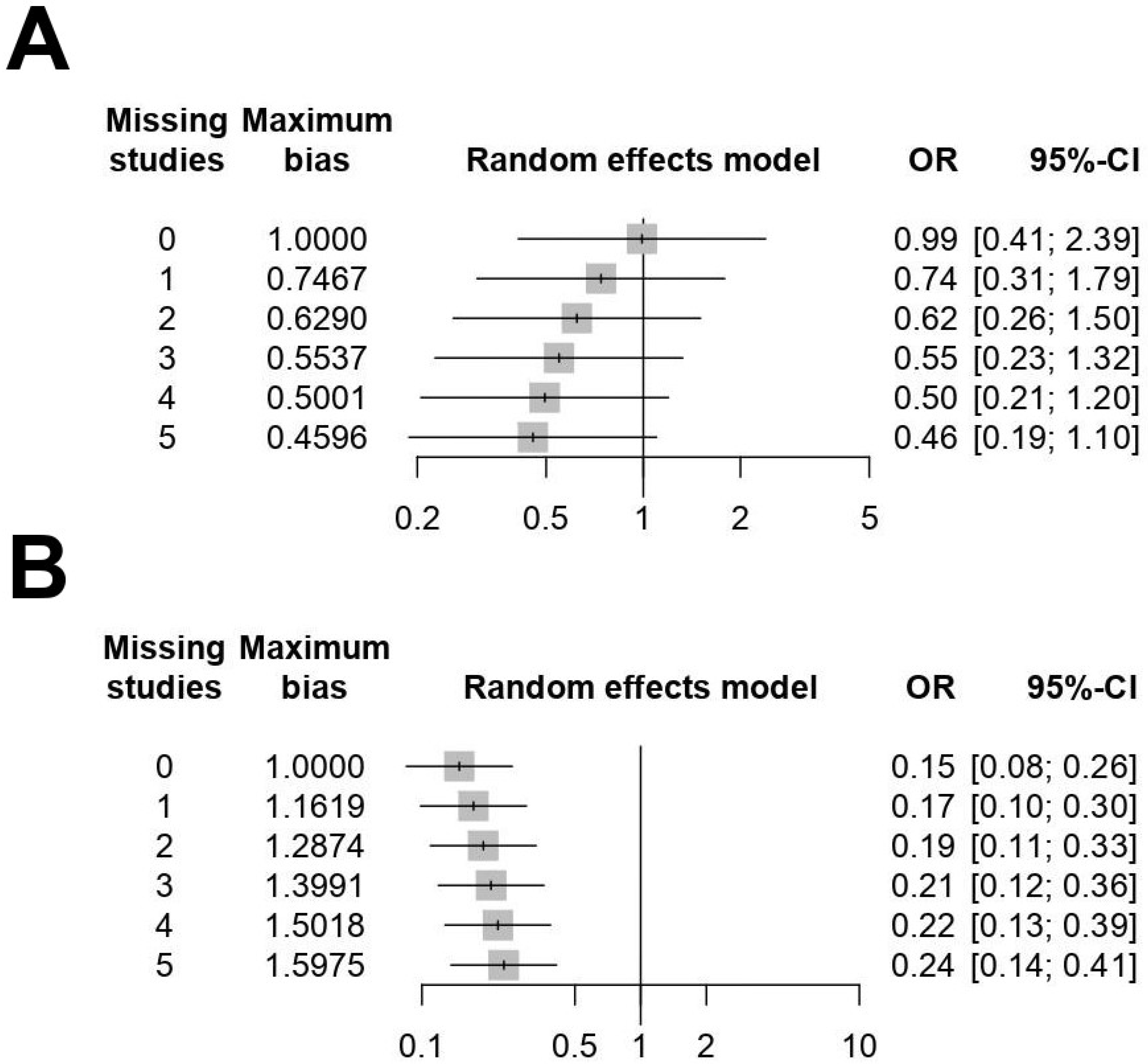
3.3. Publication Bias
3.4. Heterogeneity Assessment
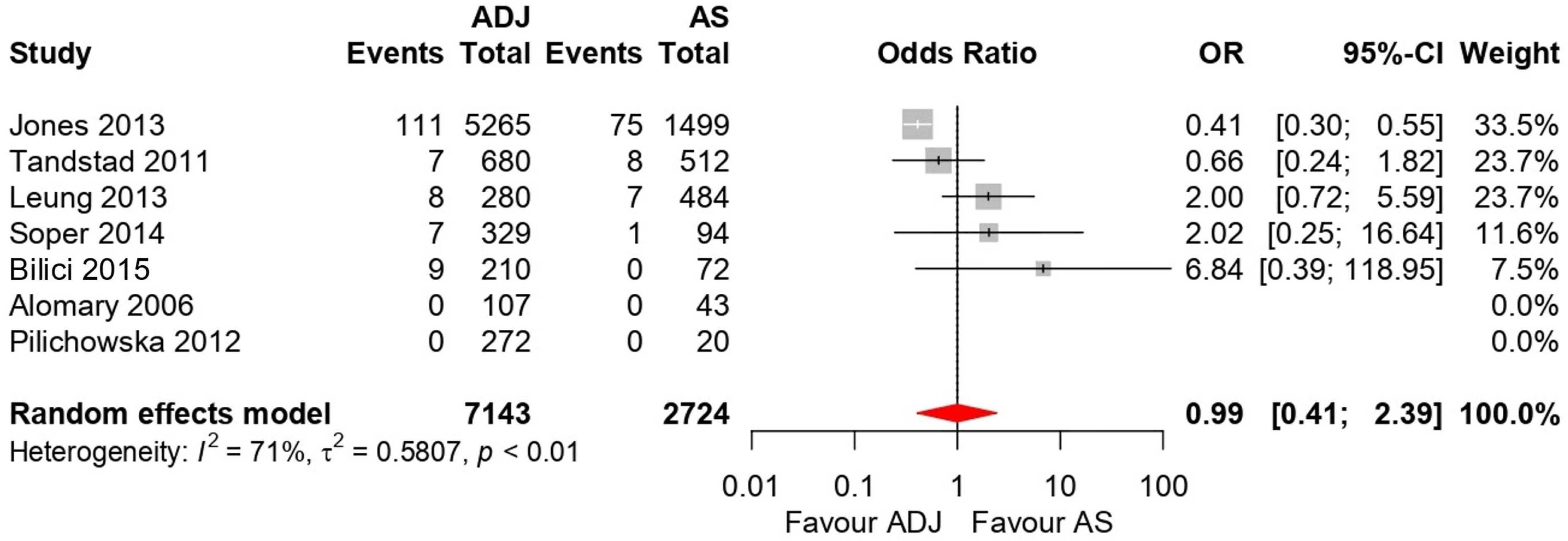
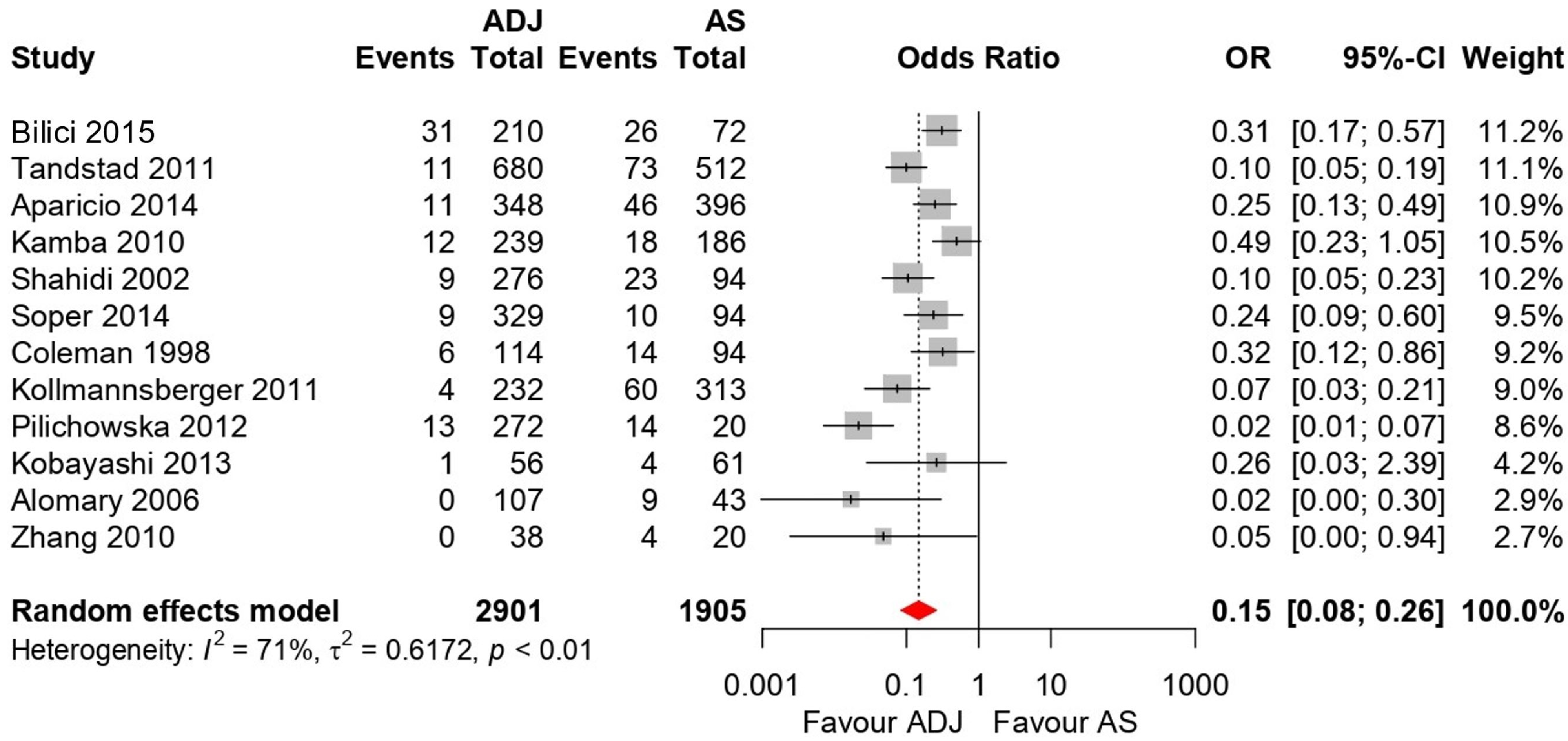
3.5. Forest Plot Results
3.5.1. Five-Year OS
3.5.2. Five-Year RFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horwich, A.; Shipley, J.; Huddart, R. Testicular germ-cell cancer. Lancet 2006, 367, 754–765. [Google Scholar] [CrossRef]
- Chia, V.M.; Quraishi, S.M.; Devesa, S.S.; Purdue, M.P.; Cook, M.B.; McGlynn, K.A. International trends in the incidence of testicular cancer, 1973–2002. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1151–1159. [Google Scholar] [CrossRef]
- Chung, P.; Mayhew, L.A.; Warde, P.; Winquist, E.; Lukka, H. Management of stage I seminomatous testicular cancer: A systematic review. Clin. Oncol. (R. Coll. Radiol.) 2010, 22, 6–16. [Google Scholar] [CrossRef]
- Travis, L.B.; Curtis, R.E.; Storm, H.; Hall, P.; Holowaty, E.; Van Leeuwen, F.E.; Kohler, B.A.; Pukkala, E.; Lynch, C.F.; Andersson, M.; et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J. Natl. Cancer Inst. 1997, 89, 1429–1439. [Google Scholar] [CrossRef]
- Krege, S.; Beyer, J.; Souchon, R.; Albers, P.; Albrecht, W.; Algaba, F.; Bamberg, M.; Bodrogi, I.; Bokemeyer, C.; Cavallin-Ståhl, E.; et al. European consensus conference on diagnosis and treatment of germ cell cancer: A report of the second meeting of the European Germ Cell Cancer Consensus group (EGCCCG): Part I. Eur. Urol. 2008, 53, 478–496. [Google Scholar] [CrossRef]
- Motzer, R.J.; Agarwal, N.; Beard, C.; Bolger, G.B.; Boston, B.; Carducci, M.A.; Choueiri, T.K.; Figlin, R.A.; Fishman, M.; Hancock, S.L.; et al. NCCN clinical practice guidelines in oncology: Testicular cancer. J. Natl. Compr. Cancer Netw. JNCCN 2009, 7, 672–693. [Google Scholar] [CrossRef]
- Cohn-Cedermark, G.; Stahl, O.; Tandstad, T. Surveillance vs. adjuvant therapy of clinical stage I testicular tumors—A review and the SWENOTECA experience. Andrology 2015, 3, 102–110. [Google Scholar] [CrossRef]
- Cummins, S.; Yau, T.; Huddart, R.; Dearnaley, D.; Horwich, A. Surveillance in stage I seminoma patients: A long-term assessment. Eur. Urol. 2010, 57, 673–678. [Google Scholar] [CrossRef]
- Ribal, M.J.; Cornford, P.; Briganti, A.; Knoll, T.; Gravas, S.; Babjuk, M.; Harding, C.; Breda, A.; Bex, A.; Rassweiler, J.J.; et al. European Association of Urology Guidelines Office Rapid Reaction Group: An Organisation-wide Collaborative Effort to Adapt the European Association of Urology Guidelines Recommendations to the Coronavirus Disease 2019 Era☆. Eur. Urol. 2020, 78, 21–28. [Google Scholar] [CrossRef]
- Petrelli, F.; Coinu, A.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Lonati, V.; Barni, S. Surveillance or Adjuvant Treatment With Chemotherapy or Radiotherapy in Stage I Seminoma: A Systematic Review and Meta-Analysis of 13 Studies. Clin. Genitourin. Cancer 2015, 13, 428–434. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Alomary, I.; Samant, R.; Gallant, V. Treatment of stage I seminoma: A 15-year review. Urol. Oncol. 2006, 24, 180–183. [Google Scholar] [CrossRef]
- Aparicio, J.; Maroto, P.; García Del Muro, X.; Sánchez-Muñoz, A.; Gumà, J.; Margelí, M.; Sáenz, A.; Sagastibelza, N.; Castellano, D.; Arranz, J.A.; et al. Prognostic factors for relapse in stage I seminoma: A new nomogram derived from three consecutive, risk-adapted studies from the Spanish Germ Cell Cancer Group (SGCCG). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 2173–2178. [Google Scholar] [CrossRef]
- Bilici, A.; Ozturk, T.; Turkmen, E.; Odabas, H.; Cihan, S.; Selcukbiricik, F.; Erdogan, B.; Urakci, Z.; Kandemir, N.; Bayoglu, I.V.; et al. Treatment preferences in stage IA and IB testicular seminoma: Multicenter study of Anatolian Society of Medical Oncology. World J. Urol. 2015, 33, 1613–1622. [Google Scholar] [CrossRef]
- Coleman, J.M.; Coleman, R.E.; Turner, A.R.; Radstone, C.R.; Champion, A.E. The management and clinical course of testicular seminoma: 15 years’ experience at a single institution. Clin. Oncol. (R. Coll. Radiol.) 1998, 10, 237–241. [Google Scholar] [CrossRef]
- Jones, G.; Arthurs, B.; Kaya, H.; Macdonald, K.; Qin, R.; Fairbanks, R.K.; Lamoreaux, W.T.; Jawed, I.; Tward, J.D.; Martincic, D.; et al. Overall survival analysis of adjuvant radiation versus observation in stage I testicular seminoma: A surveillance, epidemiology, and end results (SEER) analysis. Am. J. Clin. Oncol. 2013, 36, 500–504. [Google Scholar] [CrossRef]
- Kamba, T.; Kamoto, T.; Okubo, K.; Teramukai, S.; Kakehi, Y.; Matsuda, T.; Ogawa, O. Outcome of different post-orchiectomy management for stage I seminoma: Japanese multi-institutional study including 425 patients. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2010, 17, 980–987. [Google Scholar] [CrossRef]
- Kobayashi, K.; Saito, T.; Kitamura, Y.; Nobushita, T.; Kawasaki, T.; Hara, N.; Takahashi, K. Oncological outcomes in patients with stage I testicular seminoma and nonseminoma: Pathological risk factors for relapse and feasibility of surveillance after orchiectomy. Diagn. Pathol. 2013, 8, 57. [Google Scholar] [CrossRef]
- Kollmannsberger, C.; Tyldesley, S.; Moore, C.; Chi, K.N.; Murray, N.; Daneshmand, S.; Black, P.; Duncan, G.; Hayes-Lattin, B.; Nichols, C. Evolution in management of testicular seminoma: Population-based outcomes with selective utilization of active therapies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 808–814. [Google Scholar] [CrossRef]
- Leung, E.; Warde, P.; Jewett, M.; Panzarella, T.; O’Malley, M.; Sweet, J.; Moore, M.; Sturgeon, J.; Gospodarowicz, M.; Chung, P. Treatment burden in stage I seminoma: A comparison of surveillance and adjuvant radiation therapy. BJU Int. 2013, 112, 1088–1095. [Google Scholar] [CrossRef]
- Pilichowska, M.; Pęczkowski, P.; Rosińska-Okrasa, D.; Trzaska, B.; Skowrońska-Gardas, A.; Demkow, T. Treatment of stage I seminoma: 25 years of experience. Contemp. Oncol. 2012, 16, 104–107. [Google Scholar] [CrossRef]
- Shahidi, M.; Norman, A.R.; Dearnaley, D.P.; Nicholls, J.; Horwich, A.; Huddart, R.A. Late recurrence in 1263 men with testicular germ cell tumors. Multivariate analysis of risk factors and implications for management. Cancer 2002, 95, 520–530. [Google Scholar] [CrossRef]
- Soper, M.S.; Hastings, J.R.; Cosmatos, H.A.; Slezak, J.M.; Wang, R.; Lodin, K. Observation versus adjuvant radiation or chemotherapy in the management of stage I seminoma: Clinical outcomes and prognostic factors for relapse in a large US cohort. Am. J. Clin. Oncol. 2014, 37, 356–359. [Google Scholar] [CrossRef]
- Tandstad, T.; Smaaland, R.; Solberg, A.; Bremnes, R.M.; Langberg, C.W.; Laurell, A.; Stierner, U.K.; Ståhl, O.; Cavallin-Ståhl, E.K.; Klepp, O.H.; et al. Management of seminomatous testicular cancer: A binational prospective population-based study from the Swedish norwegian testicular cancer study group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 719–725. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Liu, Z.W.; Zhou, F.J.; Han, H.; Qin, Z.K.; Ye, Y.L.; Li, Y.H.; Hou, G.L.; Zhang, Z.L. Ten-year experience in the treatment of clinical stage I seminoma. Chin. J. Cancer 2010, 29, 92–95. [Google Scholar]
- Chauhan, S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed. J. 2020, 43, 334–340. [Google Scholar] [CrossRef]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S.; et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef]
- Czeisler, M.; Marynak, K.; Clarke, K.E.N.; Salah, Z.; Shakya, I.; Thierry, J.M.; Ali, N.; McMillan, H.; Wiley, J.F.; Weaver, M.D.; et al. Delay or Avoidance of Medical Care Because of COVID-19-Related Concerns—United States, June 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1250–1257. [Google Scholar] [CrossRef]
- Kang, D.H.; Cho, K.S.; Moon, Y.J.; Chung, D.Y.; Jung, H.D.; Lee, J.Y. Effect of neoadjuvant chemotherapy on overall survival of patients with T2-4aN0M0 bladder cancer: A systematic review and meta-analysis according to EAU COVID-19 recommendation. PLoS ONE 2022, 17, e0267410. [Google Scholar] [CrossRef]
- Moon, Y.J.; Cho, K.S.; Jeong, J.Y.; Chung, D.Y.; Kang, D.H.; Jung, H.D.; Lee, J.Y. Effects of intravesical BCG maintenance therapy duration on recurrence rate in high-risk non-muscle invasive bladder cancer (NMIBC): Systematic review and network meta-analysis according to EAU COVID-19 recommendations. PLoS ONE 2022, 17, e0273733. [Google Scholar] [CrossRef]
- Escudero-Ávila, R.; Rodríguez-Castaño, J.D.; Osman, I.; Fernandez, F.; Medina, R.; Vargas, B.; Japón-Rodríguez, M.; Sancho, P.; Perez-Valderrama, B.; Praena-Fernández, J.M.; et al. Active surveillance as a successful management strategy for patients with clinical stage I germ cell testicular cancer. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2019, 21, 796–804. [Google Scholar] [CrossRef]
- Nayan, M.; Jewett, M.A.; Hosni, A.; Anson-Cartwright, L.; Bedard, P.L.; Moore, M.; Hansen, A.R.; Chung, P.; Warde, P.; Sweet, J.; et al. Conditional Risk of Relapse in Surveillance for Clinical Stage I Testicular Cancer. Eur. Urol. 2017, 71, 120–127. [Google Scholar] [CrossRef]

| Study | Year | Study Type | Number of Patients | 5-year OS % | 5-year RFS % | Median F/U Months | Quality Assessment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | AS | ADJ | AS | ADJ | AS | ADJ | |||||||
| Total | CT | RT | |||||||||||
| Coleman [15] | 1998 | Retrospective | 239 | 94 | 144 | - | 144 | NA | NA | 85.1 | 95.8 | NA | 7 |
| Shahidi [22] | 2002 | Retrospective | 370 | 94 | 276 | - | 276 | NA | NA | 75.5 | 96.7 | 122 | 6 |
| Alomary [12] | 2006 | Retrospective | 150 | 43 | 107 | - | 107 | 100 | 100 | 79 | 100 | 54 | 6 |
| Kamba [17] | 2010 | Retrospective | 425 | 186 | 239 | 57 | 182 | NA | NA | 90 | 95 | 52.5 | 7 |
| Zhang [25] | 2010 | Retrospective | 50 | 20 | 30 | 30 | - | NA | NA | 80 | 100 | NA | 7 |
| Kollmannsberger [19] | 2011 | Retrospective | 545 | 313 | 232 | 73 | 159 | NA | NA | 80.7 | 98 | 33/33/65 (AS/CT/RT) | 7 |
| Tandstad [24] | 2011 | Prospective | 1192 | 512 | 680 | 199 | 481 | 98.4 | 99 | 85.7 | 98.4 | 60/41/73 (AS/CT/RT) | 8 |
| Pilichowska [21] | 2012 | Retrospective | 292 | 20 | 272 | 200 | 75 | 100 | 100 | 31.4 | 95.2 | 76.5 | 6 |
| Jones [16] | 2013 | Retrospective | 6764 | 1499 | 5265 | - | 5265 | 95 | 97.9 | NA | NA | 91 | 6 |
| Kobayashi [18] | 2013 | Retrospective | 118 | 61 | 56 | - | 56 | NA | NA | 93.4 | 98.2 | 67/174 (AS/RT) | 7 |
| Leung [20] | 2013 | Retrospective | 764 | 484 | 280 | - | 280 | 98.6 | 97.2 | NA | NA | 79/102 (AS/RT) | 8 |
| Aparicio [13] | 2014 | Prospective | 744 | 396 | 348 | 348 | - | NA | NA | 88.3 | 96.8 | 80 | 7 |
| Soper [23] | 2014 | Retrospective | 423 | 94 | 329 | - | 329 | 98.8 | 98 | 89.2 | 97.2 | 62/90 (AS/RT) | 7 |
| Bilici [14] | 2015 | Retrospective | 282 | 72 | 210 | 80 | 130 | 100 | 95.7 | 64.2 | 93.8 | 38.5 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.H.; Cho, K.S.; Jeong, J.Y.; Moon, Y.J.; Chung, D.Y.; Jung, H.D.; Lee, J.Y. Surveillance versus Adjuvant Treatment with Chemotherapy or Radiotherapy for Stage I Seminoma: A Systematic Review and Meta-Analysis According to EAU COVID-19 Recommendations. Medicina 2022, 58, 1514. https://doi.org/10.3390/medicina58111514
Kang DH, Cho KS, Jeong JY, Moon YJ, Chung DY, Jung HD, Lee JY. Surveillance versus Adjuvant Treatment with Chemotherapy or Radiotherapy for Stage I Seminoma: A Systematic Review and Meta-Analysis According to EAU COVID-19 Recommendations. Medicina. 2022; 58(11):1514. https://doi.org/10.3390/medicina58111514
Chicago/Turabian StyleKang, Dong Hyuk, Kang Su Cho, Jae Yong Jeong, Young Joon Moon, Doo Yong Chung, Hae Do Jung, and Joo Yong Lee. 2022. "Surveillance versus Adjuvant Treatment with Chemotherapy or Radiotherapy for Stage I Seminoma: A Systematic Review and Meta-Analysis According to EAU COVID-19 Recommendations" Medicina 58, no. 11: 1514. https://doi.org/10.3390/medicina58111514
APA StyleKang, D. H., Cho, K. S., Jeong, J. Y., Moon, Y. J., Chung, D. Y., Jung, H. D., & Lee, J. Y. (2022). Surveillance versus Adjuvant Treatment with Chemotherapy or Radiotherapy for Stage I Seminoma: A Systematic Review and Meta-Analysis According to EAU COVID-19 Recommendations. Medicina, 58(11), 1514. https://doi.org/10.3390/medicina58111514







