Fatal Form of COVID-19 in a Young Male Bodybuilder Anabolic Steroid Using: The First Autopsied Case
Abstract
1. Introduction
2. Materials and Methods
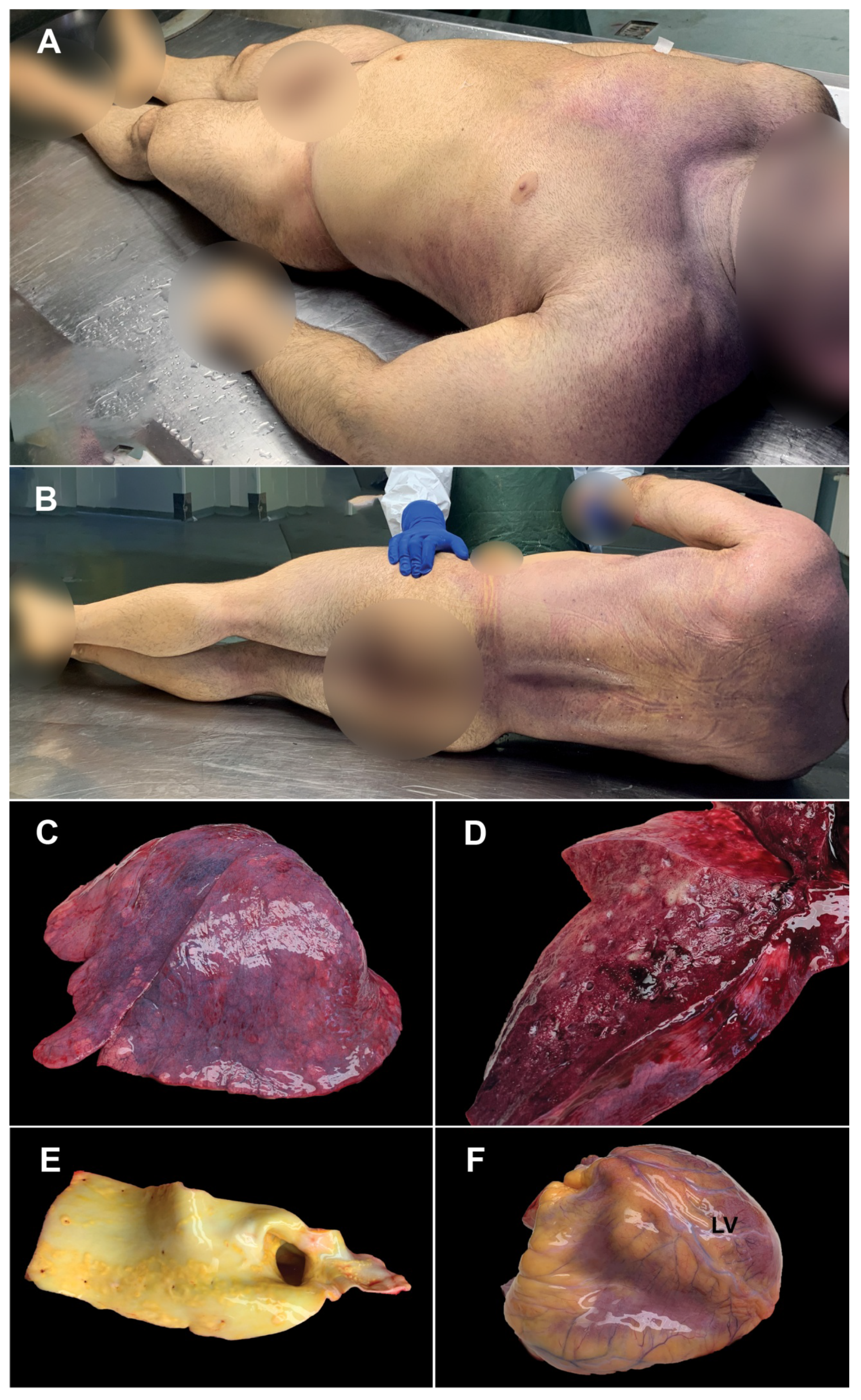
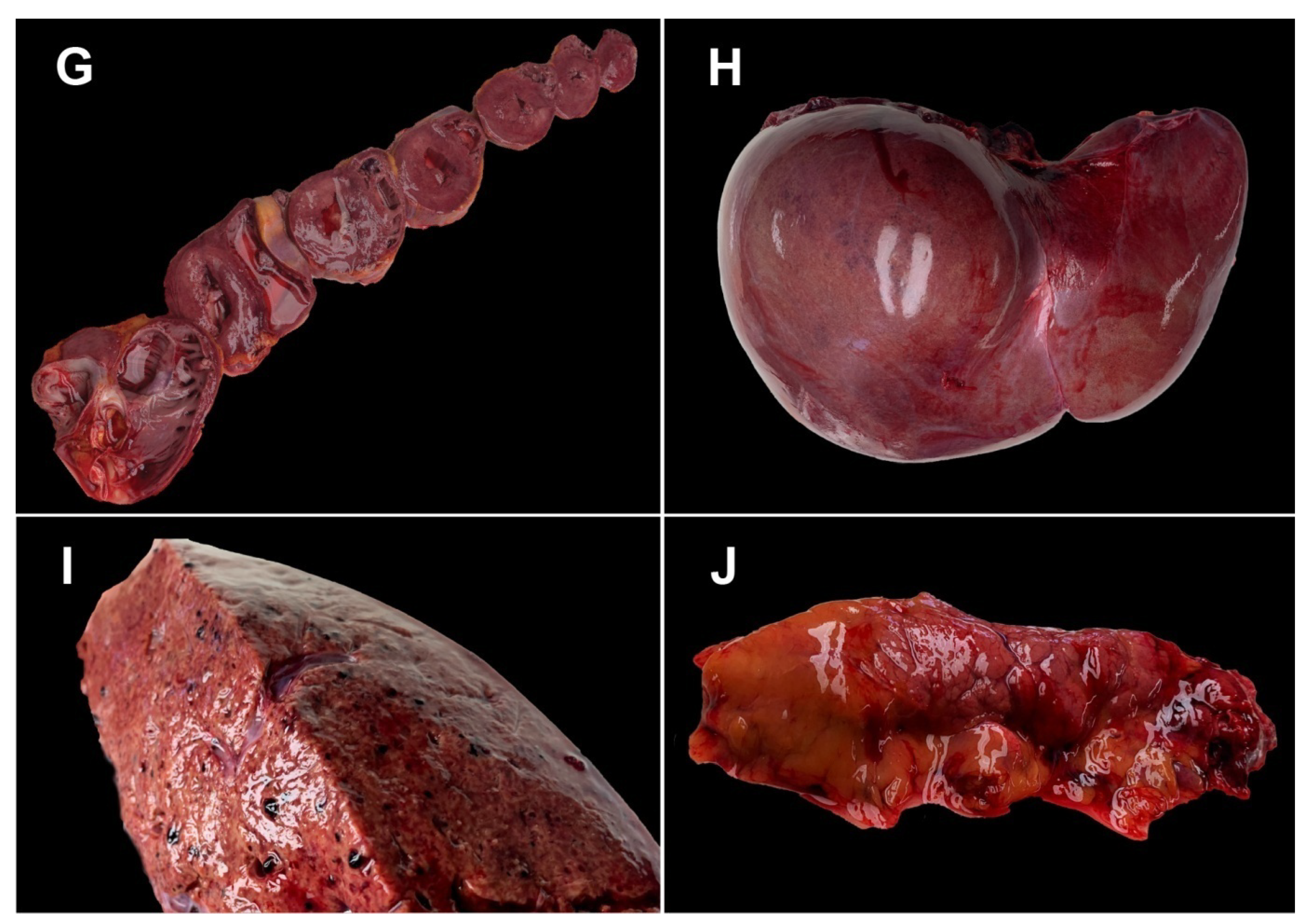
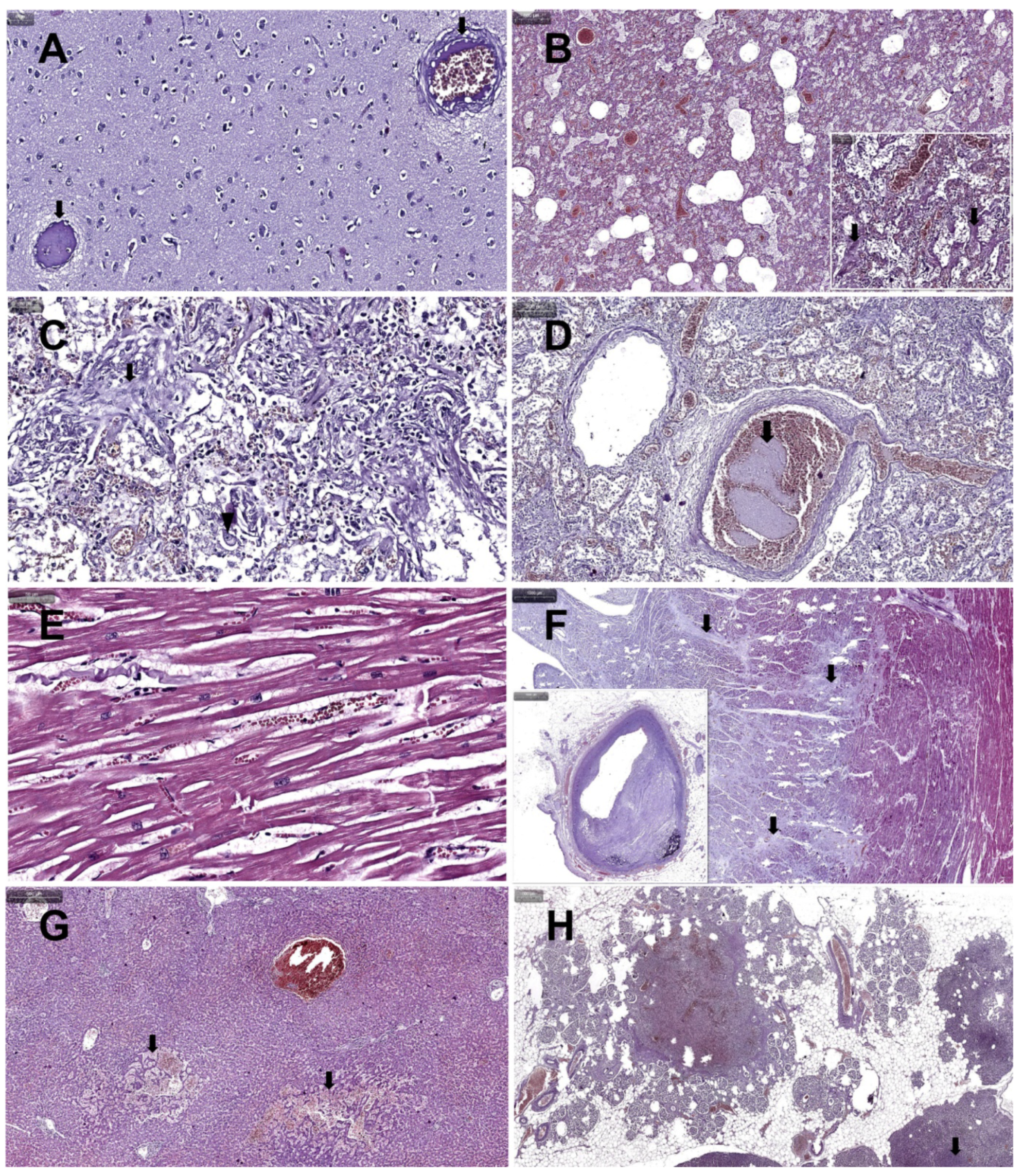
3. Case Presentation
- -
- 4 days before death, the patient presented infectious symptomatology with sudden onset (altered general condition, fever 38 °C, curvature, nausea, vomiting, dry cough, dyspnea whose intensity increased progressively); about 15 min before death, the patient presented anxiety, obnubilation, severe dyspnea; death occurred suddenly;
- -
- 3 days before the onset of the mentioned symptomatology, the patient encountered COVID-19-positive subjects in the gym room;
- -
- during the 4 days of illness, the patient self-medicated with paracetamol (3–4 tablets/day), ibuprofen 400 mg (1–2 tablets/day), aspirin in antiplatelet doses (2 tablets/day); the patient refused to be medically evaluated during the illness;
- -
- cardiovascular pathology was noted in the patient’s family history—essential arterial hypertension in both parents and polyglobulia (father);
- -
- the patient’s personal pathological history was essential hypertension grade II (highest value of Blood Pressure 180/90 mmHg), controlled under monotherapy with bisoprolol 5 mg/day, frequent palpitations with the subjective perception of an irregular heart rhythm, tachycardia, visible apex shock in the left V intercostal space on the medio-clavicular line in orthostatism;
- -
- the patient had been constantly practicing strength sports (powerlifting and bodybuilding) for the past 16 years. To improve his performance, the patient had used AASs in a continuous cycle since the age of 18. In the last 6 months before his death, the patient had used Sustanon 250 mg/mL (3 doses/week: Monday, Wednesday, Friday), Nandrolone decanoate 100 mg/mL (administration rate identical to Sustanon), Trenbolone acetate 100 mg/mL alternatively with Methenolon enanthate (2 doses/week). The patient also used, more or less regularly, fast-acting insulin, growth hormones and derivatives (GHRP-6 peptides, ipamorelin, vermotropin), multivitamins, omega 3-6-9 fatty acids (3–4 capsules daily), linseed oil, high molecular weight carbohydrate powder, isolated protein powder 2 g/body kg/day, creatine, caffeine-based energy drinks. In the last 2–3 years, the patient was doing 5 bodybuilding workouts/week, without cardio type workouts, and his diet consisted of 3 main meals with a total nutritional value of less than 3000 kcal.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonnecaze, A.K.; O’Connor, T.; Aloi, J.A. Characteristics and Attitudes of Men Using Anabolic Androgenic Steroids (AAS): A Survey of 2385 Men. Am. J. Men’s Health 2020, 14, 1557988320966536. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Gradisnik, S.; Green, R.; Brenu, E.; Weatherby, R. Anabolic androgenic steroids effects on the immune system: A review. J. Open Life Sci. 2009, 4, 19–33. [Google Scholar] [CrossRef]
- Althobaiti, Y.S.; Alzahrani, M.S.; Alhumayani, S.M.; Assiry, S.A.; Aljuaid, H.F.; Algarni, M.A. Potential Association between the Use of Anabolic Steroids and COVID-19 Infection. Healthcare 2022, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.; Lin, E.M.; Goren, A.; Wambier, C.G. Potential risk for developing severe COVID-19 disease among anabolic steroid users. BMJ Case Rep. 2021, 14, e241572. [Google Scholar] [CrossRef] [PubMed]
- Langton, D.J.; Bourke, S.C.; Lie, B.A.; Reiff, G.; Natu, S.; Darlay, R.; Burn, J.; Echevarria, C. The influence of HLA genotype on the severity of COVID-19 infection. HLA 2021, 98, 14–22. [Google Scholar] [CrossRef]
- Fischer, J.C.; Schmidt, A.G.; Bölke, E.; Uhrberg, M.; Keitel, V.; Feldt, T.; Jensen, B.; Häussinger, D.; Adams, O.; Schneider, E.M.; et al. Association of HLA genotypes, AB0 blood type and chemokine receptor 5 mutant CD195 with the clinical course of COVID-19. Eur. J. Med. Res. 2021, 26, 107. [Google Scholar] [CrossRef]
- Novelli, A.; Andreani, M.; Biancolella, M.; Liberatoscioli, L.; Passarelli, C.; Colona, V.L.; Rogliani, P.; Leonardis, F.; Campana, A.; Carsetti, R.; et al. HLA allele frequencies and susceptibility to COVID-19 in a group of 99 Italian patients. HLA 2020, 96, 610–614. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Franco, A.; Barrios, Y.; Cáceres, J.J.; Solé-Violán, J.; Perez, A.; Marcos Y Ramos, J.A.; Ramos-Gómez, L.; Ojeda, N.; et al. HLA genetic polymorphisms and prognosis of patients with COVID-19. Med. Intensiv. 2021, 45, 96–103. [Google Scholar] [CrossRef]
- Correale, P.; Mutti, L.; Pentimalli, F.; Baglio, G.; Saladino, R.E.; Sileri, P.; Giordano, A. HLA-B*44 and C*01 Prevalence Correlates with Covid19 Spreading across Italy. Int. J. Mol. Sci. 2020, 21, 5205. [Google Scholar] [CrossRef]
- Warren, R.L.; Birol, I. Retrospective in silico HLA predictions from COVID-19 patients reveal alleles associated with disease prognosis. MedRxiv 2020. [Google Scholar] [CrossRef]
- Weiner, J.; Suwalski, P.; Holtgrewe, M.; Rakitko, A.; Thibeault, C.; Müller, M.; Patriki, D.; Quedenau, C.; Krüger, U.; Ilinsky, V.; et al. Increased risk of severe clinical course of COVID-19 in carriers of HLA-C*04:01. EClinicalMedicine 2021, 40, 101099. [Google Scholar] [CrossRef]
- Brejová, B.; Boršová, K.; Hodorová, V.; Čabanová, V.; Reizigová, L.; Paul, E.D.; Čekan, P.; Klempa, B.; Nosek, J.; Vinař, T. A SARS-CoV-2 mutant from B.1.258 lineage with ∆H69/∆V70 deletion in the Spike protein circulating in Central Europe in the fall 2020. Virus Genes 2021, 57, 556–560. [Google Scholar] [CrossRef]
- Dotson, J.L.; Brown, R.T. The history of the development of anabolic-androgenic steroids. Pediatr. Clin. N. Am. 2007, 54, 761–769. [Google Scholar] [CrossRef]
- Cinislioglu, A.E.; Cinislioglu, N.; Demirdogen, S.O.; Sam, E.; Akkas, F.; Altay, M.S.; Utlu, M.; Sen, I.A.; Yildirim, F.; Kartal, S.; et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: A prospective study. Andrology 2022, 10, 24–33. [Google Scholar] [CrossRef]
- Apaydin, T.; Sahin, B.; Dashdamirova, S.; Dincer Yazan, C.; Elbasan, O.; Ilgin, C.; Bilgin, H.; Cam, H.K.; Bahramzada, G.; Kucuk, A.; et al. The association of free testosterone levels with coronavirus disease 2019. Andrology 2022, 10, 1038–1046. [Google Scholar] [CrossRef]
- Samuel, R.M.; Majd, H.; Richter, M.N.; Ghazizadeh, Z.; Zekavat, S.M.; Navickas, A.; Ramirez, J.T.; Asgharian, H.; Simoneau, C.R.; Bonser, L.R.; et al. Androgen Signaling Regulates SARS-CoV-2 Receptor Levels and Is Associated with Severe COVID-19 Symptoms in Men. Cell Stem Cell 2020, 27, 876–889. [Google Scholar] [CrossRef]
- Mendenhall, C.L.; Grossman, C.J.; Roselle, G.A.; Hertelendy, Z.; Ghosn, S.J.; Lamping, K.; Martin, K. Anabolic steroid effects on immune function: Differences between analogues. J. Steroid Biochem. Mol. Biol. 1990, 37, 71–76. [Google Scholar] [CrossRef]
- Brenu, E.W.; McNaughton, L.; Marshall-Gradisnik, S.M. Is there a potential immune dysfunction with anabolic androgenic steroid use?: A review. Mini Rev. Med. Chem. 2011, 11, 438–445. [Google Scholar] [CrossRef]
- Massart, S.; Redivo, B.; Flamion, E.; Mandiki, S.N.; Falisse, E.; Milla, S.; Kestemont, P. The trenbolone acetate affects the immune system in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2015, 163, 109–120. [Google Scholar] [CrossRef]
- Ohlander, S.J.; Varghese, B.; Pastuszak, A.W. Erythrocytosis Following Testosterone Therapy. Sex Med. Rev. 2018, 6, 77–85. [Google Scholar] [CrossRef]
- Albano, G.D.; Amico, F.; Cocimano, G.; Liberto, A.; Maglietta, F.; Esposito, M.; Rosi, G.L.; Di Nunno, N.; Salerno, M.; Montana, A. Adverse Effects of Anabolic-Androgenic Steroids: A Literature Review. Healthcare 2021, 9, 97. [Google Scholar] [CrossRef]
- Perry, J.C.; Schuetz, T.M.; Memon, M.D.; Faiz, S.; Cancarevic, I. Anabolic Steroids and Cardiovascular Outcomes: The Controversy. Cureus 2020, 12, e9333. [Google Scholar] [CrossRef]
- Chang, S.; Münster, A.B.; Gram, J.; Sidelmann, J.J. Anabolic Androgenic Steroid Abuse: The Effects on Thrombosis Risk, Coagulation, and Fibrinolysis. Semin. Thromb. Hemost. 2018, 44, 734–746. [Google Scholar] [CrossRef]
- Poor, H.D. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest 2021, 160, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Keane, G.; Dorman, T. Fatal pulmonary thromboembolism in asymptomatic COVID-19. Ir. J. Med. Sci. 2022, 191, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2021, 11, 604000. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive effects of androgens on the immune system. Cell Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef]
- Calabrese, L.H.; Kleiner, S.M.; Barna, B.P.; Skibinski, C.I.; Kirkendall, D.T.; Lahita, R.G.; Lombardo, J.A. The effects of anabolic steroids and strength training on the human immune response. Med. Sci. Sports Exerc. 1989, 21, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.N.; Wyder, D.; Spasic, D.; Herren, T. Severe rhinovirus pneumonia in a young woman taking performance-enhancing drugs. BMJ Case Rep. 2016, 2016, bcr2015213836. [Google Scholar] [CrossRef]
- Herr, A.; Rehmert, G.; Kunde, K.; Gust, R.; Gries, A. 30-jähriger Bodybuilder mit septischem Schock und ARDS bei Abusus anabolandrogener Steroide [A thirty-year old bodybuilder with septic shock and ARDS from abuse of anabolic steroids]. Anaesthesist 2002, 51, 557–563. [Google Scholar] [CrossRef]
- Bertozzi, G.; Sessa, F.; Maglietta, F.; Cipolloni, L.; Salerno, M.; Fiore, C.; Fortarezza, P.; Ricci, P.; Turillazzi, E.; Pomara, C. Immunodeficiency as a side effect of anabolic androgenic steroid abuse: A case of necrotizing myofasciitis. Forensic. Sci. Med. Pathol. 2019, 15, 616–621. [Google Scholar] [CrossRef]
- LoBue, S.A.; Goldman, A.; Giovane, R.A.; Carlson, S.M.; Bivona, M.; Albear, S.; LoBue, T.D. Recurrent Herpes Zoster Ophthalmicus Preceded by Anabolic Steroids and High-Dose L-Arginine. Case Rep. Ophthalmol. Med. 2020, 2020, 8861892. [Google Scholar] [CrossRef]
- Seara, F.A.C.; Olivares, E.L.; Nascimento, J.H.M. Anabolic steroid excess and myocardial infarction: From ischemia to reperfusion injury. Steroids 2020, 161, 108660. [Google Scholar] [CrossRef]
- Bagheri, S.A.; Boyer, J.L. Peliosis hepatis associated with androgenic-anabolic steroid therapy. A severe form of hepatic injury. Ann. Intern. Med. 1974, 81, 610–618. [Google Scholar] [CrossRef]
- Suchi, M.; MacMullen, C.; Thornton, P.S.; Ganguly, A.; Stanley, C.A.; Ruchelli, E.D. Histopathology of congenital hyperinsulinism: Retrospective study with genotype correlations. Pediatr. Dev. Pathol. 2003, 6, 322–333. [Google Scholar] [CrossRef]
- Brinkman, J.E.; Tariq, M.A.; Leavitt, L.; Sharma, S. Physiology, Growth Hormone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482141/ (accessed on 24 June 2022).
- Konopka, K.E.; Nguyen, T.; Jentzen, J.M.; Rayes, O.; Schmidt, C.J.; Wilson, A.M.; Farver, C.F.; Myers, J.L. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 Infection is Morphologically Indistinguishable from Other Causes of DAD. Histopathology 2020, 77, 570–578. [Google Scholar] [CrossRef]
- Valdebenito, S.; Bessis, S.; Annane, D.; Lorin de la Grandmaison, G.; Cramer-Bordé, E.; Prideaux, B.; Eugenin, E.A.; Bomsel, M. COVID-19 Lung Pathogenesis in SARS-CoV-2 Autopsy Cases. Front. Immunol. 2021, 12, 735922. [Google Scholar] [CrossRef]
- Jeican, I.I.; Gheban, D.; Barbu-Tudoran, L.; Inișca, P.; Albu, C.; Ilieș, M.; Albu, S.; Vică, M.L.; Matei, H.V.; Tripon, S.; et al. Respiratory Nasal Mucosa in Chronic Rhinosinusitis with Nasal Polyps versus COVID-19: Histopathology, Electron Microscopy Analysis and Assessing of Tissue Interleukin-33. J. Clin. Med. 2021, 10, 4110. [Google Scholar] [CrossRef]
- Müller, J.A.; Groß, R.; Conzelmann, C.; Krüger, J.; Merle, U.; Steinhart, J.; Weil, T.; Koepke, L.; Bozzo, C.P.; Read, C.; et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021, 3, 149–165. [Google Scholar] [CrossRef]
- Bouquegneau, A.; Erpicum, P.; Grosch, S.; Habran, L.; Hougrand, O.; Huart, J.; Krzesinski, J.-M.; Misset, B.; Hayette, M.-P.; Delvenne, P.; et al. COVID-19-associated Nephropathy Includes Tubular Necrosis and Capillary Congestion, with Evidence of SARS-CoV-2 in the Nephron. Kidney360 2021, 2, 639–652. [Google Scholar] [CrossRef]
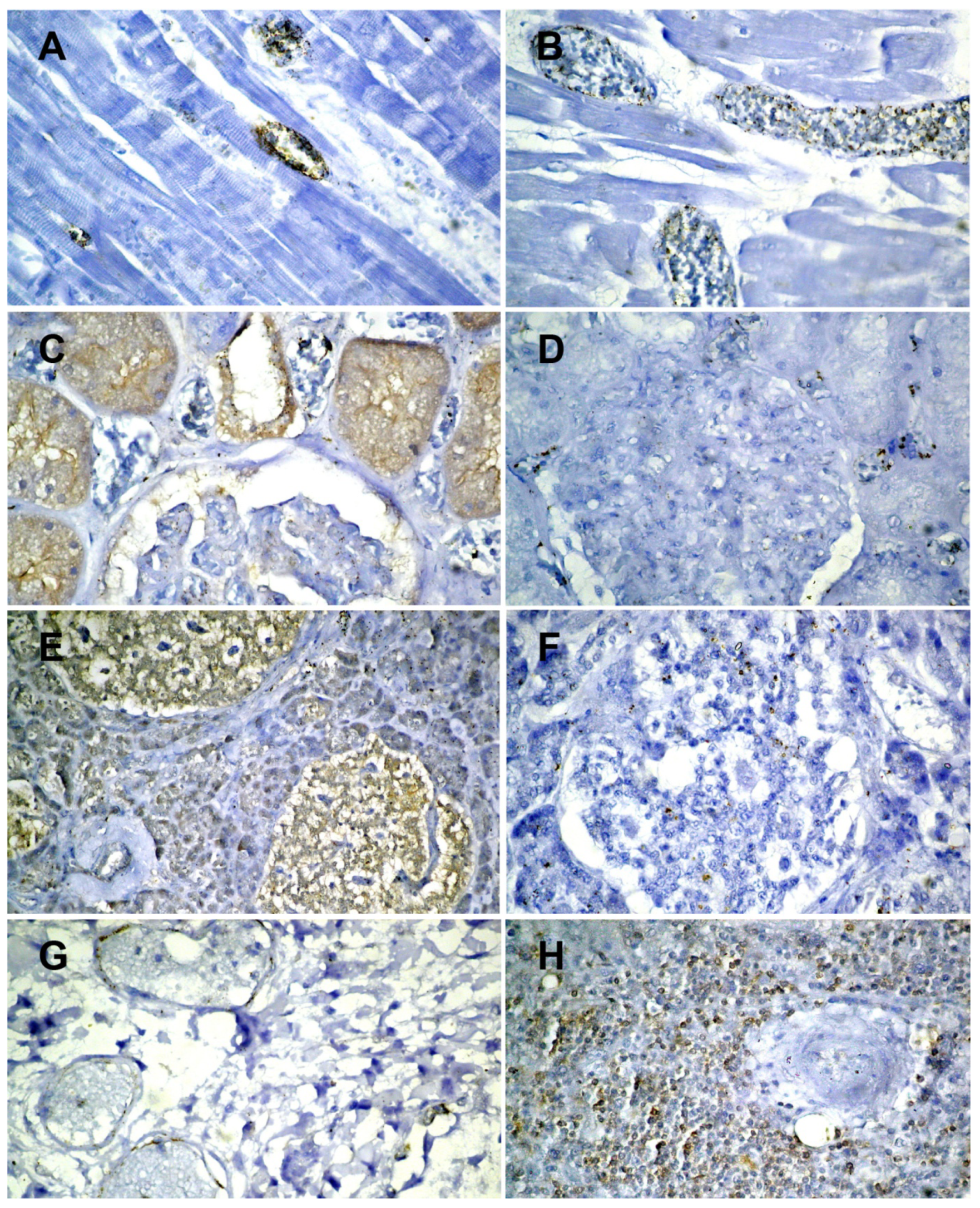
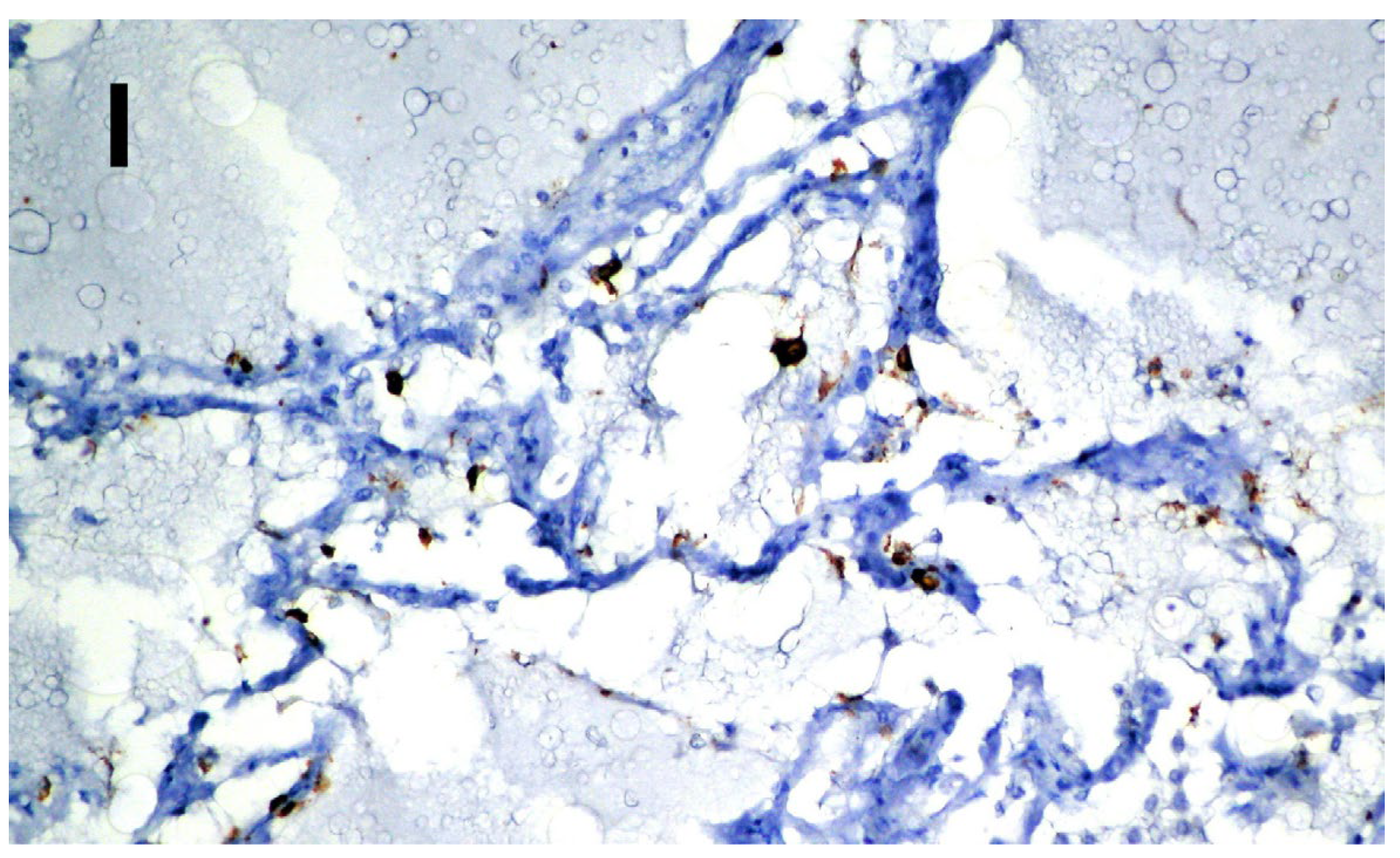
| ACE 2 | SARS-CoV-2 | |
|---|---|---|
| Brain | Negative | Negative |
| Myocardium | Positive on the vascular endothelium (Figure 3A) | Positive on the vascular endothelium (Figure 3B) |
| Lung | Positive on the vascular endothelium (Figure 3G) | Positive on alveolocytes (Figure 3I) |
| Spleen | Positive on the lymphocytes in the follicles (Figure 3H) | Negative |
| Kidneys | Positive on proximal tubules and on the endothelium of the corresponding arteriole (Figure 3C) | Positive on intertubular capillaries (Figure 3D) |
| Liver | Positive on the biliary epithelium, the endothelium of the centrilobular venules | Negative |
| Pancreas | Positive on the islets of Langerhans (beta cells) (Figure 3E) | Positive on the islets of Langerhans (beta cells) (Figure 3F) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siserman, C.V.; Jeican, I.I.; Gheban, D.; Anton, V.; Mironescu, D.; Șușman, S.; Vică, M.L.; Lazăr, M.; Aluaș, M.; Toader, C.; et al. Fatal Form of COVID-19 in a Young Male Bodybuilder Anabolic Steroid Using: The First Autopsied Case. Medicina 2022, 58, 1373. https://doi.org/10.3390/medicina58101373
Siserman CV, Jeican II, Gheban D, Anton V, Mironescu D, Șușman S, Vică ML, Lazăr M, Aluaș M, Toader C, et al. Fatal Form of COVID-19 in a Young Male Bodybuilder Anabolic Steroid Using: The First Autopsied Case. Medicina. 2022; 58(10):1373. https://doi.org/10.3390/medicina58101373
Chicago/Turabian StyleSiserman, Costel Vasile, Ionuț Isaia Jeican, Dan Gheban, Vlad Anton, Daniela Mironescu, Sergiu Șușman, Mihaela Laura Vică, Mihaela Lazăr, Maria Aluaș, Corneliu Toader, and et al. 2022. "Fatal Form of COVID-19 in a Young Male Bodybuilder Anabolic Steroid Using: The First Autopsied Case" Medicina 58, no. 10: 1373. https://doi.org/10.3390/medicina58101373
APA StyleSiserman, C. V., Jeican, I. I., Gheban, D., Anton, V., Mironescu, D., Șușman, S., Vică, M. L., Lazăr, M., Aluaș, M., Toader, C., & Albu, S. (2022). Fatal Form of COVID-19 in a Young Male Bodybuilder Anabolic Steroid Using: The First Autopsied Case. Medicina, 58(10), 1373. https://doi.org/10.3390/medicina58101373









