Potential Association between Subclinical Hypothyroidism and Childhood Migraine
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Clinical Evaluation
2.3. Body Mass Index
2.4. Thyroid Function Tests
2.5. Radiological and Histological Examination
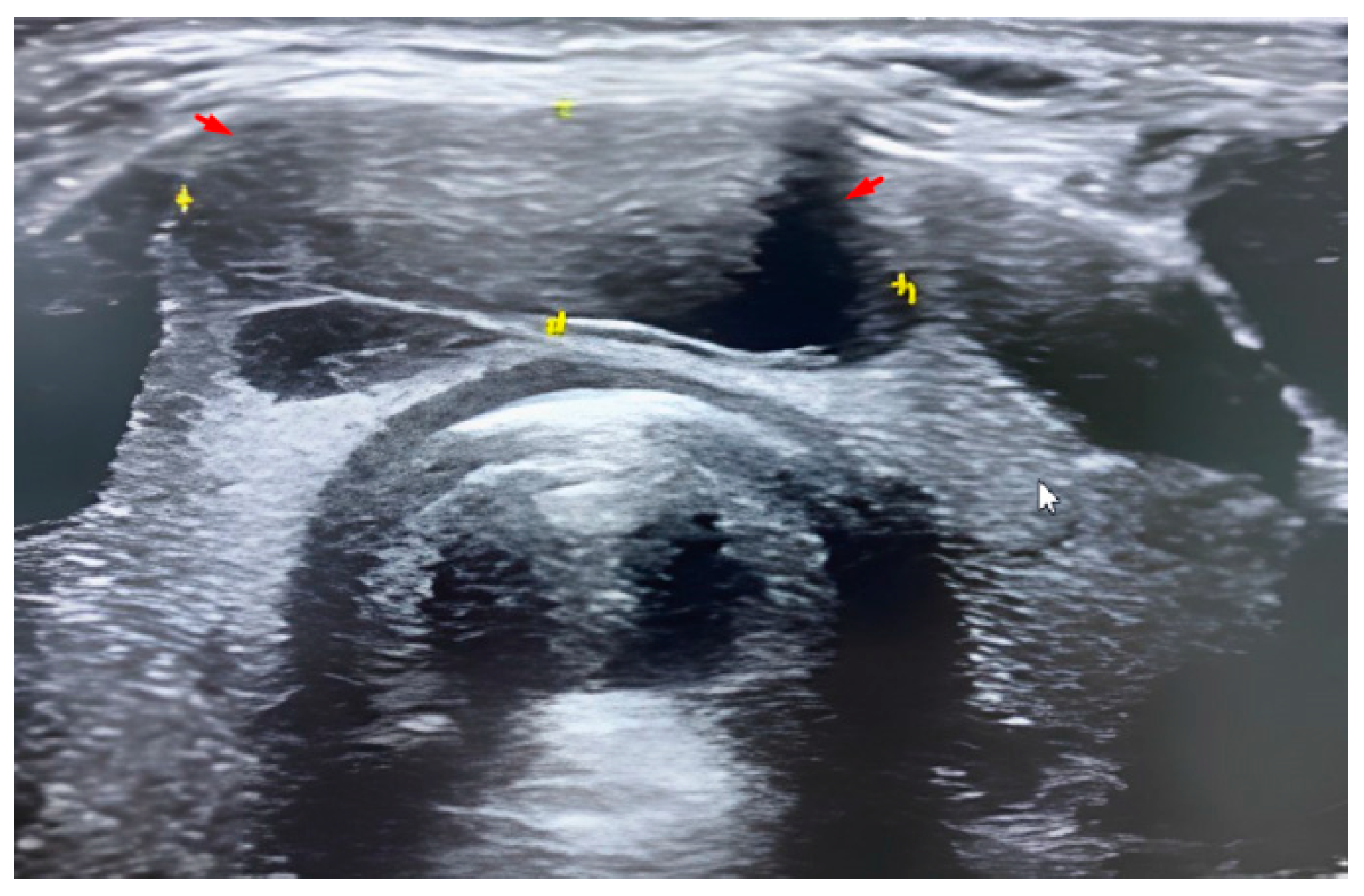
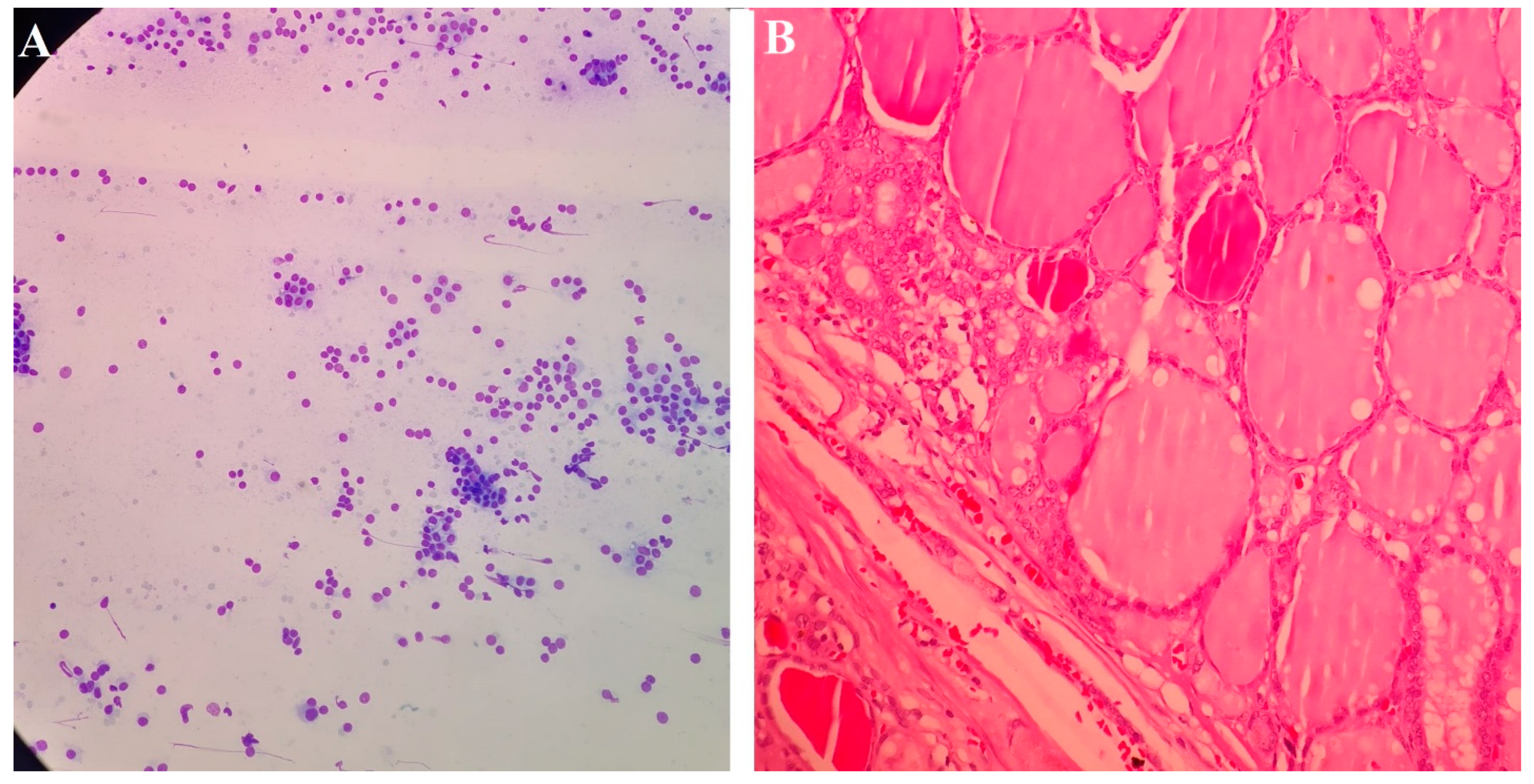
2.6. Methodology of FNA
2.7. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Al Khalili, Y.; Chopra, P. Migraine Headache in Childhood. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557813/ (accessed on 20 January 2022).
- Jain, S.; Malinowski, M.; Chopra, P.; Varshney, V.; Deer, T.R. Intrathecal drug delivery for pain management: Recent advances and future developments. Expert Opin. Drug Deliv. 2019, 16, 815–822. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, H.L.; Kabbouche, M.A.; Kacperski, J.; Hershey, A.D. Treatment of pediatric migraine. Curr. Treat. Options Neurol. 2015, 17, 326. [Google Scholar] [CrossRef] [PubMed]
- Kandt, R.S.; Levine, R.M. Headache and acute illness in children. J. Child Neurol. 1987, 2, 22–27. [Google Scholar] [CrossRef][Green Version]
- Crisafulli, G.; Aversa, T.; Zirilli, G.; Pajno, G.B.; Corica, D.; De Luca, F.; Wasniewska, M. Subclinical Hypothyroidism in Children: When a Replacement Hormonal Treatment Might Be Advisable. Front. Endocrinol. 2019, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Karmisholt, J.; Andersen, S.; Laurberg, P. Variation in thyroid function in subclinical hypothyroidism: Importance of clinical follow-up and therapy. Eur. J. Endocrinol. 2011, 164, 317–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paoli-Valeri, M.; Mamán-Alvarado, D.; Jiménez-López, V.; Arias-Ferreira, A.; Bianchi, G.; Arata-Bellabarba, G. Frequency of subclinical hypothyroidism among healthy children and those with neurological conditions in the state of Mérida, Venezuela. Investig. Clin. 2003, 44, 209–218. [Google Scholar]
- Wu, T.; Flowers, J.W.; Tudiver, F.; Wilson, J.L.; Punyasavatsut, N. Subclinical thyroid disorders and cognitive performance among adolescents in the United States. BMC Pediatr. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Monzani, A.; Prodam, F.; Bellone, S.; Bona, G. Subclinical Hypothyrodism. In Thyroid Diseases in Childhood: Recent Advances from Basic Science to Clinical Practice; Bona, G., De Luca, F., Monzani, A., Eds.; Springer International Publishing: Basel, Switzerland, 2015; pp. 195–202. [Google Scholar]
- Wasniewska, M.; Salerno, M.; Cassio, A.; Aversa, T.; Zirilli, G.; Capalbo, D.; Bal, M.; Mussa, A.; De Luca, F. Prospective evaluation of the natural course of idiopathic subclinical hypothyroidism in childhood and adolescence. Eur. J. Endocrinol. 2009, 160, 417–421. [Google Scholar] [CrossRef]
- De Luca, F.; Wasniewska, M.; Zirilli, G.; Aversa, T.; Arrigo, T. At the end of a two-year follow-up elevated TSH levels normalize or remain unchanged in most the children with subclinical hypothyroidism. Ital. J. Pediatr. 2010, 36, 11. [Google Scholar] [CrossRef] [PubMed]
- Wasniewska, M.; Aversa, T.; Salerno, M.; Corrias, A.; Messina, M.F.; Mussa, A.; Capalbo, D.; De Luca, F.; Valenzise, M. Five-year prospective evaluation of thyroid function in girls with subclinical mild hypothyroidism of different etiology. Eur. J. Endocrinol. 2015, 173, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Emad, E.; Mousa, M.; Shehta, N. Migraine and Subclinical Hypothyroidism: A Possible Co-morbidity. Zagazig Univ. Med. J. 2022, 28, 379–388. [Google Scholar] [CrossRef]
- Abou Elmaaty, A.A.; Flifel, M.E.; Belal, T.; Zarad, C.A. Migraine and tension headache comorbidity with hypothyroidism in Egypt. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 78. [Google Scholar] [CrossRef]
- Rubino, E.; Rainero, I.; Garino, F.; Vicentini, C.; Govone, F.; Vacca, A.; Gai, A.; Gentile, S.; Govone, G.; Ragazzoni, F.; et al. Subclinical hypothyroidism is associated with migraine: A case-control study. Cephalalgia 2019, 39, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lima Carvalho, M.F.; de Medeiros, J.S.; Valença, M.M. Headache in recent onset hypothyroidism: Prevalence, characteristics and outcome after treatment with levothyroxine. Cephalalgia 2017, 37, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.T.; Pinney, S.M.; Xie, C.; Herrick, R.L.; Bai, Y.; Buckholz, J.; Martin, V.T. Headache disorders may be a risk factor for the development of new onset hypothyroidism. Headache 2017, 57, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mirouliaei, M.; Fallah, R.; Bashardoost, N.; Partovee, M.; Ordooei, M. Efficacy of levothyroxine in migraine headaches in children with subclinical hypothyroidism. Iran. J. Child Neurol. 2012, 6, 23–26. [Google Scholar] [PubMed]
- Iwasaki, Y.; Kinoshita, M.; Ikeda, K.; Takamiya, K.; Shiojima, T. Thyroid function in patients with chronic headache. Int. J. Neurosci. 1991, 57, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.M.; Vodo, S.; Buonocore, M. Pain and thyroid hormones. Neurol. Sci. 2013, 34, 1501–1508. [Google Scholar] [CrossRef]
- Fallah, R.; Mirouliaei, M.; Bashardoost, N.; Partovee, M. Frequency of subclinical hypothyroidism in 5- to 15-year-old children with migraine headache. J. Pediatr. Endocrinol. Metab. 2012, 25, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Ekici, B.; Cebeci, A.N. The debate on the link between subclinical hypothyroidism and childhood migraine: Is initial endocrinological evaluation necessary for children with migraine? Acta Neurol. Belg. 2015, 115, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018, 38, 1–211. [CrossRef]
- Hershey, A.D.; Powers, S.W.; Vockell, A.L.; LeCates, S.; Segers, A.; Kabbouche, M.; Hershey, A. Development of a patient-based grading scale for PedMIDAS. Cephalalgia 2004, 24, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Edlbeck, A.; Kirby, M.; Goday, P. Ideal body weight in children. Nutr. Clin. Pract. 2007, 22, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ghalli, I.; Salah, N.; Hussien, F.; Erfan, M.; El-Ruby, M.; Mazen, I.; Sabry, M.; Abd El-Razik, M.; Saad, M.; Hossney, L.; et al. Egyptian growth curves 2002 for infants, children and adolescents. In Crescere Nel Mondo; Sartorio, A., Buckler, J.M.H., Marazzi, N., Eds.; Ferring Publisher: Milan, Italy, 2008. [Google Scholar]
- Barlow, S.E.; The Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. S4), S164–S192. [Google Scholar] [CrossRef] [PubMed]
- Lo, F.S. Reference Intervals for Laboratory Tests and Procedures. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., Joseph St. Geme, J.W., III, Eds.; Saunders: Philadelphia, PA, USA, 2019; p. ch748. [Google Scholar]
- Luster, M.; Aktolun, C.; Amendoeira, I.; Barczyński, M.; Bible, K.C.; Duntas, L.H.; Elisei, R.; Handkiewicz-Junak, D.; Hoffmann, M.; Jarząb, B.; et al. European perspective on 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: Proceedings of an interactive international symposium. Thyroid 2019, 29, 7–26. [Google Scholar]
- Khan, H.; Shah, P.; Bhat, H.; Imran, A. Association of hypothyroidism in patients with migraine and tension- type headache disorders in Kashmir, North India. Neurol. Asia 2015, 20, 257–261. [Google Scholar]
- Rainero, I.; Rubino, E.; Vicentini, C.; Garino, F.; Ragazzoni, F.; Pinessi, L.; Limone, P. Prevalence of migraine in subclinical hypothyroidism: A case-control study. J. Headache Pain 2015, 16 (Suppl. S1), A81. [Google Scholar] [CrossRef][Green Version]
- Toprak, D.; Demirkukan, K.; Ellidokuz, H. Is it important to test thyroid function tests in migraineurs? Turk. J. Fam. Med. Prim. Care 2007, 4, 47–51. [Google Scholar]
- Hagen, K.; Bjoro, T.; Zwart, J.; Vatten, L.; Stovner, L.J.; Bovim, G. Low headache prevalence amongst women with high TSH values. Eur. J. Neurol. 2001, 8, 693–699. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Meschi, T.; Cervellin, G.; Borghi, L. Homocysteine and migraine. A narrative review. Clin. Chim. Acta 2014, 433, 5–11. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Cao, X.; Liu, C.; Xie, Y. Association between plasma homocysteine status and hypothyroidism: A meta-analysis. J. Neurol. Sci. 2014, 7, 4544–4553. [Google Scholar]
- Lee, H.; Li, C.; Hammerstad, S.; Stefan, M.; Tomer, Y. Immunogenetics of autoimmune thyroid diseases: A comprehensive review. J. Autoimmun. 2015, 64, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rainero, I.; Fasano, E.; Rubino, E.; Rivoiro, C.; Valfrè, W.; Gallone, S.; Savi, L.; Gentile, S.; Giudice, R.L.; De Martino, P.; et al. Association between migraine and HLA-DRB1 gene polymorphisms. J. Headache Pain 2005, 6, 185–187. [Google Scholar] [CrossRef]
- Arumugam, M.; Parthasarathy, V. Reduction of CD4+ CD25+ regulatory T-cells in migraine: Is migraine an autoimmune disorder? J. Neuroimmunol. 2016, 290, 54–59. [Google Scholar] [CrossRef]
- Sarchielli, P.; Alberti, A.; Baldi, A.; Coppola, F.; Rossi, C.; Pierguidi, L.; Floridi, A.; Calabresi, P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 2006, 46, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Song, M.; Kim, Y.; Park, K.; Ryu, C. Changes in thyroid peroxidase activity in response to various chemicals. J. Environ. Monit. 2012, 14, 2121–2126. [Google Scholar] [CrossRef]
- Vermeer, L.; Gregory, E.; Winter, M.; McCarson, K.; Berman, N.E.J. Exposure to bisphenol A exacerbates migraine-like behaviors in a multibehavior model of rat migraine. Toxicol. Sci. 2014, 137, 416–427. [Google Scholar] [CrossRef]
- Saif, E.; El-Belbasy, R.; Hammour, Z.E.; Mohamed, M. Prevalence of migraine, its effect, and some comorbid psychiatric disorders among female medical students at Al-Azhar University in Cairo. Al-Azhar Assiut Med. J. 2017, 15, 148. [Google Scholar]
- Danicic, J.; Inder, W.; Kotowicz, M. Impact of subclinical hypothyroidism on health-related quality of life: A narrative review. Intern. Med. J. 2021, 51, 1380–1387. [Google Scholar] [CrossRef]
- Gulseren, S.; Gulseren, L.; Hekimsoy, Z.; Cetinay, P.; Ozen, C.; Tokatlioglu, B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch. Med. Res. 2006, 37, 133–139. [Google Scholar] [CrossRef]
- Minen, M.; Begasse De Dhaem, O.; Kroon Van Diest, A.; Powers, S.; Schwedt, T.J.; Lipton, R.; Silbersweig, D. Migraine and its psychiatric comorbidities. J. Neurol. Neurosurg. Psychiatry 2016, 87, 741–749. [Google Scholar] [CrossRef]
- Bond, D.S.; Roth, J.; Nash, J.M.; Wing, R.R. Migraine and obesity: Epidemiology, possible mechanisms and the potential role of weight loss treatment. Obes. Rev. 2011, 12, e362–e371. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, D.; Raychaudhuri, M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016, 20, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, M.; Wu, M.; Wang, X.; Li, F.; Zhang, J.; You, L.; Pan, X.; Feng, W.; Wu, J.; et al. Obesity is associated with subclinical hypothyroidism in the presence of thyroid autoantibodies: A cross-sectional study. BMC Endocr. Disord. 2022, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Bona, G.; Prodam, F.; Monzani, A. Subclinical hypothyroidism in children: Natural history and when to treat. J. Clin. Res. Pediatr. Endocrinol. 2013, 5 (Suppl. S1), 23–28. [Google Scholar] [CrossRef] [PubMed]
- Metwalley, K.A.; Farghaly, H.S. Subclinical hypothyroidism in children: Updates for pediatricians. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Elmaoğulları, S.; Özalkak, Ş.; Çetinkaya, S.; Karaman, I.; Üner, Ç.; Arda, N.; Savaş-Erdeve, Ş.; Aycan, Z. Evaluation of Children and Adolescents with Thyroid Nodules: A Single Center Experience. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 276–284. [Google Scholar] [CrossRef] [PubMed]
| Migraine Cases | Control | p-Value | |
|---|---|---|---|
| N = 100 (%) | N = 100 (%) | ||
| Characteristics: | |||
| Age—mean ± SD (years) | 10.13 ± 2.37 | 10.42 ± 3.51 | 0.49 |
| Male—number (%) | 54 (54) | 52 (52) | 0.78 |
| Female—number (%) | 46 (46) | 48 (48) | |
| Family history of thyroid disorders | |||
| Negative | 97 (97) | 99 (99) | 0.621 |
| Positive | 3 (3) | 1 (1) | |
| BMI—mean ± SD | 25.5 ± 4.84 | 24.31 ± 3.31 | 0.044 * |
| BMI categories—number (%): | 0.03* | ||
| Normal | 87 (87) | 97 (97) | |
| Overweight | 8 (8) | 2 (2) | |
| Obese | 5 (5) | 1 (1) | |
| Laboratory values—mean ± SD: | |||
| FT4 (ng/dl) | 1.61 ± 0.23 | 1.65 ± 0.30 | 0.291 |
| TSH (μIU/L) | 3.94 ± 1.63 | 3.18 ± 0.99 | <0.001 * |
| Thyroid function—number (%): | <0.001 * | ||
| Normal | 81 (81) | 98 (98) | |
| Subclinical hypothyroidism | 17 (17) | 2 (2) | |
| Overt Hypothyroidism | 2 (2) | 0 (0) | |
| Goiter | 0.175 | ||
| No goiter | 96 | 99 | |
| Goiter | 4 | 1 |
| Normal Body Weight | Overweight/Obese | p-Value | |
|---|---|---|---|
| N = 87 (%) | N = 13 (%) | ||
| Thyroid function—number (%): | |||
| Normal | 80 (91.95) | 1 (7.69) | <0.001 * |
| Subclinical hypothyroidism | 7 (8.05) | 10 (76.92) | |
| Overt Hypothyroidism | 0 (0) | 2 (15.38) |
| Migraine Cases without Overt Hypothyroidism (n = 98) | |||
|---|---|---|---|
| ScH | Normal Thyroid | p-Value | |
| N = 17 (%) | N = 81 (%) | ||
| Migraine subtypes | |||
| With aura | 4 (23.5) | 22 (27.2) | 0.758 |
| Without aura | 13 (76.5) | 59 (72.8) | |
| PedMIDAS score | |||
| Mild | 3 (17.6) | 48 (59.3) | |
| Moderate | 9 (52.9) | 24 (29.6) | 0.004 * |
| Severe | 5 (29.4) | 9 (11.1) | |
| Age at onset—mean ± SD (years) | 8.93 ± 1.34 | 9.12 ± 1.20 | 0.562 |
| Duration of migraine—mean ± SD (months) | 13.83 ± 2.58 | 13.53 ± 2.61 | 0.667 |
| Normal Thyroid | ScH | Overt Hypothyroidism | p-Value | |
|---|---|---|---|---|
| Migraine with aura (N = 26) | 22 | 4 | 0 | 0.016 * |
| Control (N = 100) | 98 | 2 | 0 | |
| Migraine without aura (N = 74) | 59 | 13 | 2 | <0.001 * |
| Control (N = 100) | 98 | 2 | 0 |
| B | SE | Wald | p-Value | Odds Ratio | 95% CI for Odds Ratio | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| BMI | 0.017 | 0.004 | 11.532 | 0.001 | 1.020 | 1.009 | 1.031 |
| Age of onset (years) | −0.005 | 0.016 | 0.110 | 0.742 | 0.996 | 0.962 | 1.032 |
| Duration of illness (months) | −0.036 | 0.119 | 0.079 | 0.765 | 0.941 | 0.765 | 1.209 |
| Sex | 0.479 | 0.577 | 0.719 | 0.344 | 1.609 | 0.536 | 4.955 |
| Positive family history of thyroid illness | 0.772 | 0.570 | 1.809 | 0.188 | 2.209 | 0.704 | 6.971 |
| Aura | 0.275 | 0.165 | 2.665 | 0.980 | 1.320 | 0.938 | 1.875 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.A.E.; El-Gharieb, H.A.; Nasr, M.; Abdelhay, W.M.; Yousef, T.S.M.; El-Zamek, H.M.F.; Zidan, A.M.; Nady, M.; Abdel-Kareem, M.A.; Hasan, A. Potential Association between Subclinical Hypothyroidism and Childhood Migraine. Medicina 2022, 58, 1346. https://doi.org/10.3390/medicina58101346
Hassan MAE, El-Gharieb HA, Nasr M, Abdelhay WM, Yousef TSM, El-Zamek HMF, Zidan AM, Nady M, Abdel-Kareem MA, Hasan A. Potential Association between Subclinical Hypothyroidism and Childhood Migraine. Medicina. 2022; 58(10):1346. https://doi.org/10.3390/medicina58101346
Chicago/Turabian StyleHassan, Mohammed Abd Elmalik, Hussein Awad El-Gharieb, Mohamed Nasr, Wagih M. Abdelhay, Tahseen Samir Mohammed Yousef, Hossam M. Farid El-Zamek, Ahmed M. Zidan, Mohamed Nady, Mona A. Abdel-Kareem, and Abdulkarim Hasan. 2022. "Potential Association between Subclinical Hypothyroidism and Childhood Migraine" Medicina 58, no. 10: 1346. https://doi.org/10.3390/medicina58101346
APA StyleHassan, M. A. E., El-Gharieb, H. A., Nasr, M., Abdelhay, W. M., Yousef, T. S. M., El-Zamek, H. M. F., Zidan, A. M., Nady, M., Abdel-Kareem, M. A., & Hasan, A. (2022). Potential Association between Subclinical Hypothyroidism and Childhood Migraine. Medicina, 58(10), 1346. https://doi.org/10.3390/medicina58101346






