Loss of Expression of Antiangiogenic Protein FKBPL in Endometrioid Endometrial Carcinoma: Implications for Clinical Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples and Data
2.2. Immunohistochemical Staining
2.3. Histopathological Evaluation
2.4. Statistical Analysis
3. Results
3.1. Study Population
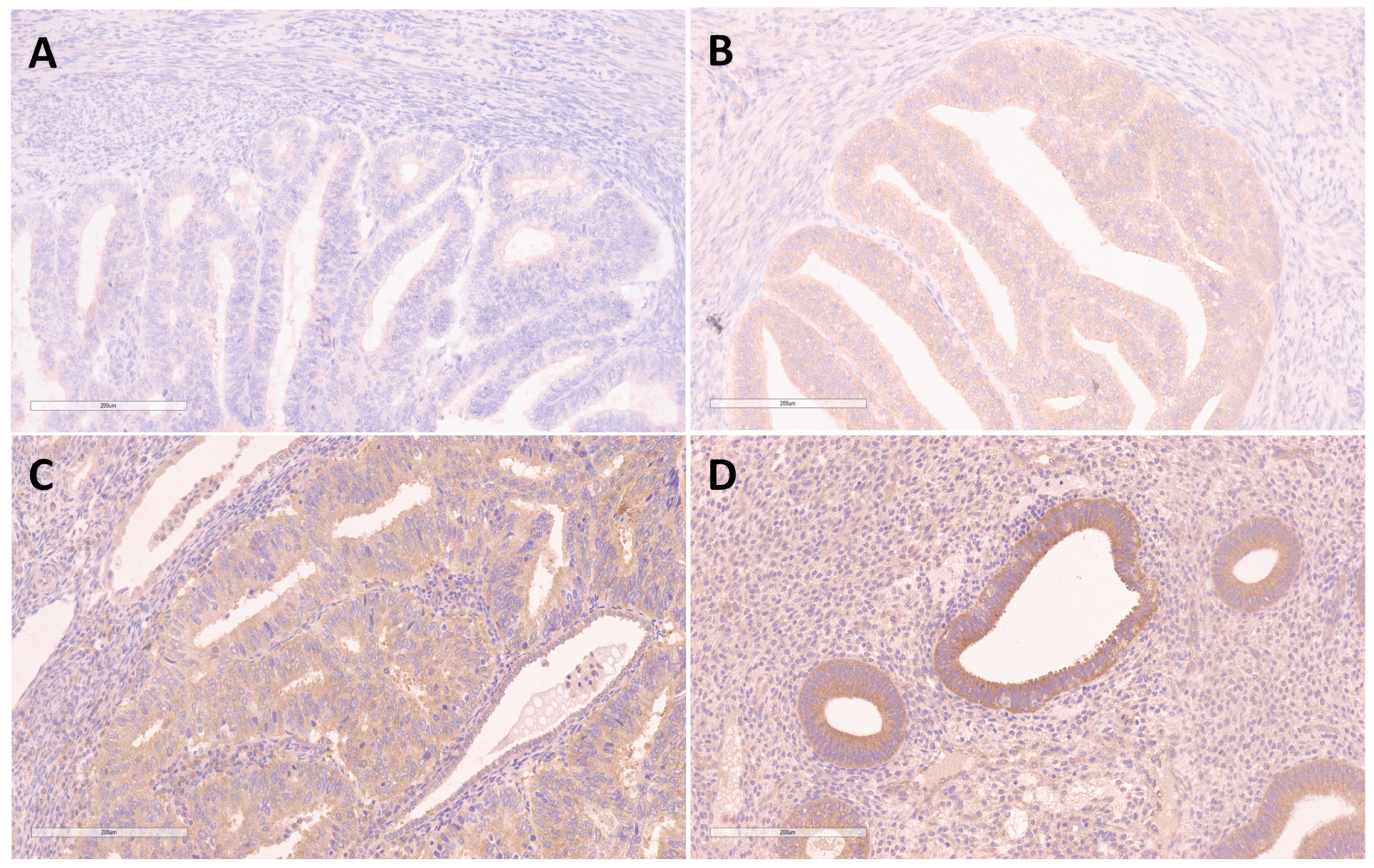
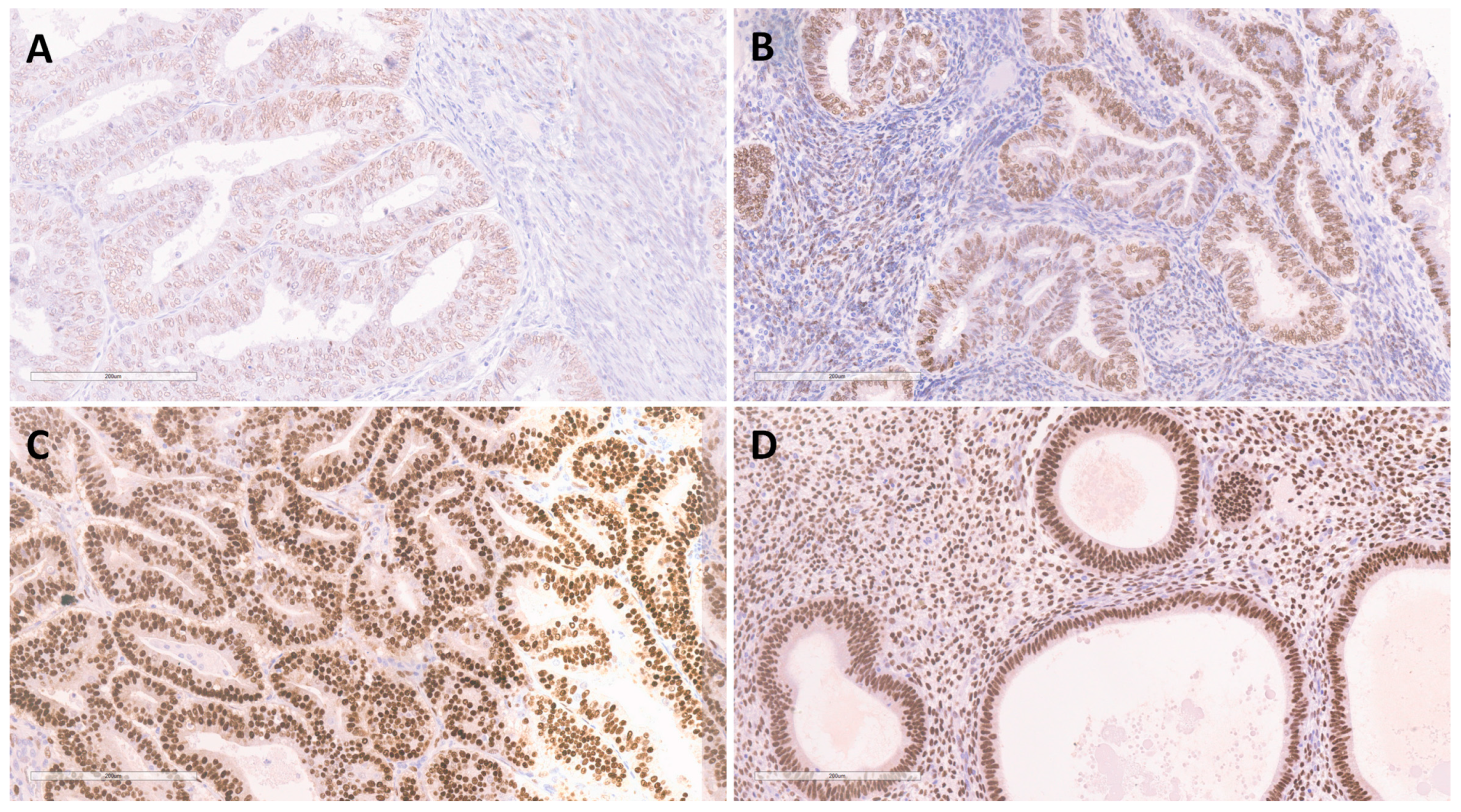
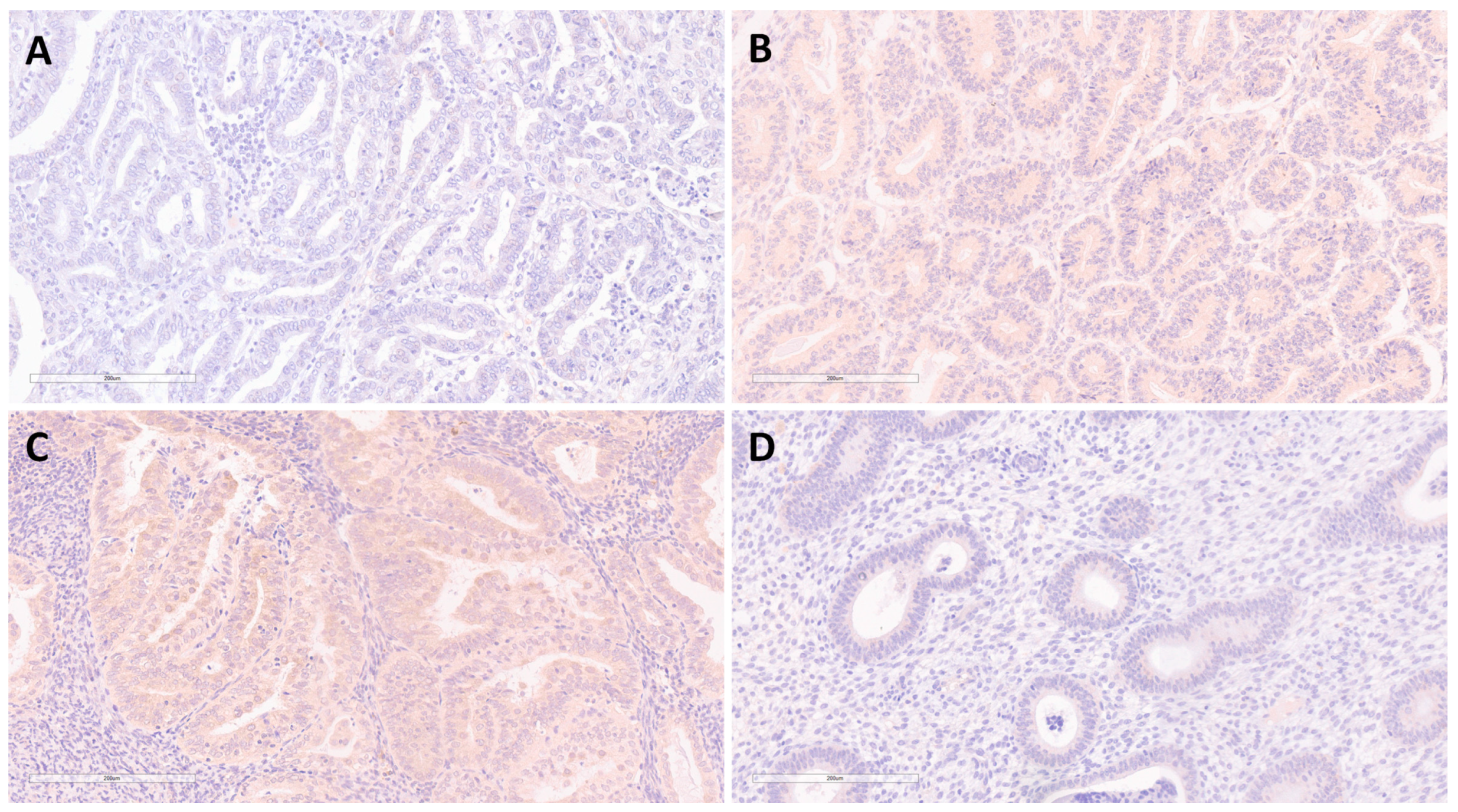
3.2. Expression of FKBPL, VEGF-A and ERα in EEC and BEH
3.2.1. Reliability of Double-Blind Reading
3.2.2. Difference of FKBPL Expression in EEC and BEH
3.2.3. Measures of Diagnostic Accuracy for FKBPL Expression
3.2.4. Expression of ERα in EEC and BEH
3.2.5. Expression of VEGF-A in EEC and BEH
4. Discussion
4.1. Expression of FKBPL in EEC and BEH
4.2. Expression of ERα and FKBPL
4.3. Expression of VEGF-A and FKBPL
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021; pp. 1–72. [Google Scholar]
- Herrington, C.S. Female Genital Tumours, 5th ed.; Organisation Mondiale de la Santé, Centre International de Recherche sur le Cancer, Eds.; World Health Organization Classification of Tumours; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Bokhman, J.V. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Sorosky, J.I. Endometrial Cancer. Obstet. Gynecol. 2012, 120, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Buhtoiarova, T.N.; Brenner, C.A.; Singh, M. Endometrial Carcinoma: Role of Current and Emerging Biomarkers in Resolving Persistent Clinical Dilemmas. Am. J. Clin. Pathol. 2016, 145, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice. Int. J. Gynecol. Pathol. 2019, 38, S64–S74. [Google Scholar] [CrossRef] [PubMed]
- Saimi, M.; Moriya, S.; Li, Z.-L.; Miyaso, H.; Nagahori, K.; Kawata, S.; Omotehara, T.; Ogawa, Y.; Hino, H.; Miyazawa, K.; et al. Cytonuclear Estrogen Receptor Alpha Regulates Proliferation and Migration of Endometrial Carcinoma Cells. Tokai J. Exp. Clin. Med. 2021, 46, 7–16. [Google Scholar] [PubMed]
- Backes, F.J.; Walker, C.J.; Goodfellow, P.J.; Hade, E.M.; Agarwal, G.; Mutch, D.; Cohn, D.E.; Suarez, A.A. Estrogen Receptor-Alpha as a Predictive Biomarker in Endometrioid Endometrial Cancer. Gynecol. Oncol. 2016, 141, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Roškar, L.; Roškar, I.; Rižner, T.L.; Smrkolj, Š. Diagnostic and Therapeutic Values of Angiogenic Factors in Endometrial Cancer. Biomolecules 2021, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Dao, F.; Levine, D.A. Angiogenesis in Endometrial Carcinoma: Therapies and Biomarkers, Current Options, and Future Perspectives. Gynecol. Oncol. 2021, 160, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Dziobek, K.; Opławski, M.; Grabarek, B.O.; Zmarzły, N.; Tomala, B.; Halski, T.; Leśniak, E.; Januszyk, K.; Brus, R.; Kiełbasiński, R.; et al. Changes in the Expression Profile of VEGF-A, VEGF-B, VEGFR-1, VEGFR-2 in Different Grades of Endometrial Cancer. Curr. Pharm. Biotechnol. 2019, 20, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.; McKeen, H.D.; Marshall, A.; Mulrane, L.; Starczynski, J.; Storr, S.J.; Lanigan, F.; Byrne, C.; Arthur, K.; Hegarty, S.; et al. FKBPL: A Marker of Good Prognosis in Breast Cancer. Oncotarget 2015, 6, 12209–12223. [Google Scholar] [CrossRef] [PubMed]
- McClements, L.; Annett, S.; Yakkundi, A.; O’Rourke, M.; Valentine, A.; Moustafa, N.; Alqudah, A.; Simões, B.M.; Furlong, F.; Short, A.; et al. FKBPL and Its Peptide Derivatives Inhibit Endocrine Therapy Resistant Cancer Stem Cells and Breast Cancer Metastasis by Downregulating DLL4 and Notch4. BMC Cancer 2019, 19, 351. [Google Scholar] [CrossRef] [PubMed]
- McKeen, H.D.; Byrne, C.; Jithesh, P.V.; Donley, C.; Valentine, A.; Yakkundi, A.; O’Rourke, M.; Swanton, C.; McCarthy, H.O.; Hirst, D.G.; et al. FKBPL Regulates Estrogen Receptor Signaling and Determines Response to Endocrine Therapy. Cancer Res. 2010, 70, 1090–1100. [Google Scholar] [CrossRef]
- Annett, S.; Moore, G.; Short, A.; Marshall, A.; McCrudden, C.; Yakkundi, A.; Das, S.; McCluggage, W.G.; Nelson, L.; Harley, I.; et al. FKBPL-Based Peptide, ALM201, Targets Angiogenesis and Cancer Stem Cells in Ovarian Cancer. Br. J. Cancer 2020, 122, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Ito, Y.M.; Kataoka, F.; Nomura, H.; Tanaka, H.; Chiyoda, T.; Hashimoto, S.; Nishimura, S.; Takano, M.; Yamagami, W.; et al. Simultaneous Analysis of the Gene Expression Profiles of Cancer and Stromal Cells in Endometrial Cancer: Stromal Gene Expression of Endometrial Cancer. Genes Chromosomes Cancer 2014, 53, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Tissue Expression of FKBPL-Staining in Endometrium—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000204315-FKBPL/tissue/endometrium (accessed on 4 August 2022).
- Expression of FKBPL in Endometrial Cancer—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000204315-FKBPL/pathology/endometrial+cancer (accessed on 4 August 2022).
- Yunokawa, M.; Yoshida, H.; Watanabe, R.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Yonemori, K.; Shimizu, C.; Fujiwara, Y.; Tamura, K. Allred Score Is a Promising Predictor of Prognosis and Medroxyprogesterone Acetate Efficacy in Patients with Endometrial Cancer. Cancer Chemother. Pharmacol. 2017, 80, 127–134. [Google Scholar] [CrossRef]
- Valentine, A.; O’Rourke, M.; Yakkundi, A.; Worthington, J.; Hookham, M.; Bicknell, R.; McCarthy, H.O.; McClelland, K.; McCallum, L.; Dyer, H.; et al. FKBPL and Peptide Derivatives: Novel Biological Agents That Inhibit Angiogenesis by a CD44-Dependent Mechanism. Clin. Cancer Res. 2011, 17, 1044–1056. [Google Scholar] [CrossRef]
- Yakkundi, A.; Bennett, R.; Hernández-Negrete, I.; Delalande, J.-M.; Hanna, M.; Lyubomska, O.; Arthur, K.; Short, A.; McKeen, H.; Nelson, L.; et al. FKBPL Is a Critical Antiangiogenic Regulator of Developmental and Pathological Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Droog, M.; Nevedomskaya, E.; Dackus, G.M.; Fles, R.; Kim, Y.; Hollema, H.; Mourits, M.J.; Nederlof, P.M.; van Boven, H.H.; Linn, S.C.; et al. Estrogen Receptor α Wields Treatment-Specific Enhancers between Morphologically Similar Endometrial Tumors. Proc. Natl. Acad. Sci. USA 2017, 114, E1316–E1325. [Google Scholar] [CrossRef] [PubMed]
- El Helali, A.; Plummer, R.; Jayson, G.C.; Coyle, V.M.; Drew, Y.; Mescallado, N.; Harris, N.; Clamp, A.R.; McCann, J.; Swaisland, H.; et al. A First-in-Human Phase I Dose-Escalation Trial of the Novel Therapeutic Peptide, ALM201, Demonstrates a Favourable Safety Profile in Unselected Patients with Ovarian Cancer and Other Advanced Solid Tumours. Br. J. Cancer 2022, 127, 92–101. [Google Scholar] [CrossRef] [PubMed]
| Variable | EEC (n = 89) | |
|---|---|---|
| Grade (%) | G1 | 40.4 (n = 36) |
| G2 | 55.1 (n = 49) | |
| G3 | 4.5 (n = 4) | |
| TNM stage (%) | T1A | 33.7 (n = 30) |
| T1B | 36 (n = 32) | |
| T2 | 20.2 (n = 18) | |
| T3 | 10.1 (n = 9) | |
| FIGO stage (%) | IA | 31.5 (n = 28) |
| IB | 36 (n = 32) | |
| II | 20.2 (n = 18) | |
| III | 11.2 (n = 10) | |
| IV | 1.1 (n = 1) | |
| Myometrial invasion depth (%) | Less than half | 37.1 (n = 33) |
| One half or more | 62 (n = 56) | |
| Lymphovascular invasion (%) | Yes | 33.7 (n = 30) |
| No | 66.3 (n = 59) | |
| Variable | Cronbach’s Alpha | Internal Consistency |
|---|---|---|
| FKBPL | 0.994 | Excellent |
| ERα (intensity) | 0.997 | Excellent |
| ERα (percentage) | 0.987 | Excellent |
| Allred score | 0.996 | Excellent |
| VEGF-A | 0.970 | Excellent |
| Variable | Benign Endometrial Hyperplasia n = 40 | Endometrioid Endometrial Carcinoma n = 89 | Significance (p) | ||
|---|---|---|---|---|---|
| FKBPL | 0/1 | 2.5% (n = 1) | 93.3% (n = 83) | <0.001 | |
| 2/3 | 97.5% (n = 39) | 6.7% (n = 6) | |||
| ERα | Intensity | 1 | 0% (n = 0) | 46.1% (n = 41) | <0.001 |
| 2/3 | 100% (n = 40) | 53.9% (n = 48) | |||
| Percentage | <5 (≤66%) | 20.0 (n = 8) | 78.7% (n = 70) | <0.001 | |
| 5 (67–100%) | 80.0 (n = 32) | 21.3% (n = 19) | |||
| Allred Score | <7 | 10% (n = 4) | 71.9% (n = 64) * | <0.001 | |
| 7/8 | 90% (n = 36) | 28.1% (n = 25) | |||
| VEGF-A | 0 | 95% (n = 38) | 57.3% (n = 51) | <0.001 | |
| 1/2 | 5% (n = 2) | 42.7% (n = 38) | |||
| Variable | Sensitivity | Positive Predictive Value | Specificity | Negative Predictive Value | Accuracy |
|---|---|---|---|---|---|
| FKBPL | 93.3% | 98.8% | 97.5% | 86.7% | 94.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obradović, D.D.; Milić, N.M.; Miladinović, N.; McClements, L.; Oprić, D.M. Loss of Expression of Antiangiogenic Protein FKBPL in Endometrioid Endometrial Carcinoma: Implications for Clinical Practice. Medicina 2022, 58, 1330. https://doi.org/10.3390/medicina58101330
Obradović DD, Milić NM, Miladinović N, McClements L, Oprić DM. Loss of Expression of Antiangiogenic Protein FKBPL in Endometrioid Endometrial Carcinoma: Implications for Clinical Practice. Medicina. 2022; 58(10):1330. https://doi.org/10.3390/medicina58101330
Chicago/Turabian StyleObradović, Danilo D., Nataša M. Milić, Nenad Miladinović, Lana McClements, and Dejan M. Oprić. 2022. "Loss of Expression of Antiangiogenic Protein FKBPL in Endometrioid Endometrial Carcinoma: Implications for Clinical Practice" Medicina 58, no. 10: 1330. https://doi.org/10.3390/medicina58101330
APA StyleObradović, D. D., Milić, N. M., Miladinović, N., McClements, L., & Oprić, D. M. (2022). Loss of Expression of Antiangiogenic Protein FKBPL in Endometrioid Endometrial Carcinoma: Implications for Clinical Practice. Medicina, 58(10), 1330. https://doi.org/10.3390/medicina58101330







