Psychogenic Pseudosyncope: Real or Imaginary? Results from a Case-Control Study in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Statistical Analysis
3. Results
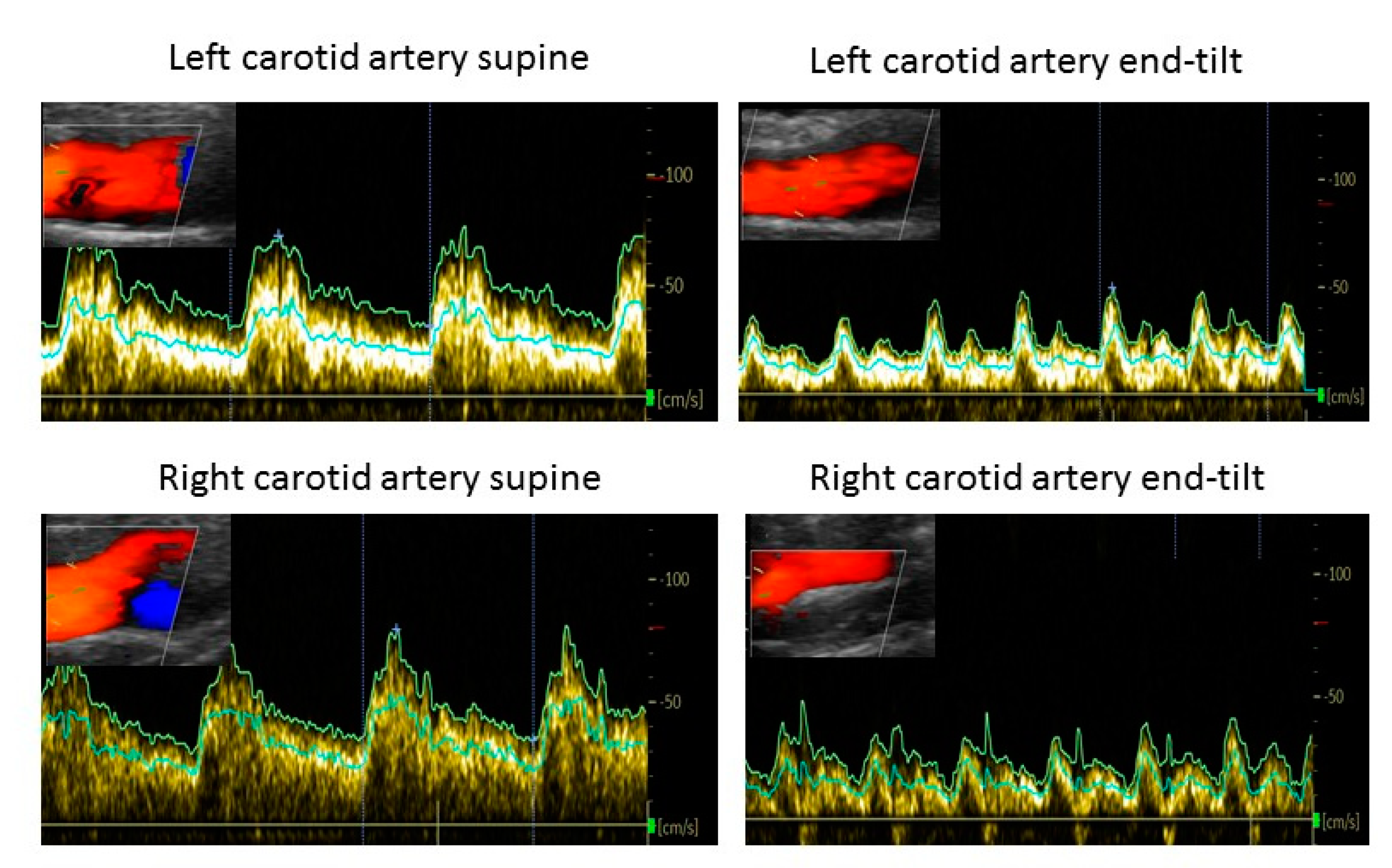
Case Example
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A.; International Chronic Fatigue Syndrome Study Group. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; van de Sande, M.I.; de Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Institute Of Medicine. Beyond Mayalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining An Illness; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Cockshell, S.J.; Mathias, J. Cognitive functioning in chronic fatigue syndrome: A meta-analysis. Psychol. Med. 2010, 40, 1253–1267. [Google Scholar] [CrossRef]
- Cvejic, R.; Birch, R.C.; Vollmer-Conna, U. Cognitive Dysfunction in Chronic Fatigue Syndrome: A Review of Recent Evidence. Curr. Rheumatol. Rep. 2016, 18, 24. [Google Scholar] [CrossRef]
- Roma, M.; Marden, C.L.; Flaherty, M.A.K.; Jasion, S.E.; Cranston, E.M.; Rowe, P.C. Impaired Health-Related Quality of Life in Adolescent Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: The Impact of Core Symptoms. Front. Pediatr. 2019, 7, 26. [Google Scholar] [CrossRef]
- Van Campen, C.L.M.C.; Verheugt, F.W.A.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020, 5, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bou-Holaigah, I.; Rowe, P.C.; Kan, J.; Calkins, H. The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA 1995, 274, 961–967. [Google Scholar] [CrossRef]
- Brignole, M.; Alboni, P.; Benditt, D.; Bergfeldt, L.; Blanc, J.; Thomsen, P.B.; Van Dijk, J.; Fitzpatrick, A.; Hohnloser, S.; Janousek, J.; et al. Guidelines on management (diagnosis and treatment) of syncope. Eur. Heart J. 2001, 22, 1256–1306. [Google Scholar] [CrossRef] [Green Version]
- Krahn, A.D.; Klein, G.J.; Norris, C.; Yee, R. The Etiology of Syncope in Patients With Negative Tilt Table and Electrophysiological Testing. Circulation 1995, 92, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, K.; Kanjwal, Y.; Karabin, B.; Grubb, B.P. Psychogenic Syncope? A Cautionary Note. Pacing Clin. Electrophysiol. 2009, 32, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-K.; Sheldon, R.S.; Benditt, D.G.; Cohen, M.I.; Forman, D.E.; Goldberger, Z.D.; Grubb, B.P.; Hamdan, M.H.; Krahn, A.D.; Link, M.S.; et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017, 136, e25–e59. [Google Scholar] [CrossRef] [Green Version]
- Walsh, K.E.; Baneck, T.; Page, R.L.; Brignole, M.; Hamdan, M.H. Psychogenic pseudosyncope: Not always a diagnosis of exclusion. Pacing Clin. Electrophysiol. 2018, 41, 480–486. [Google Scholar] [CrossRef]
- Van Campen, L.M.C.; Rowe, P.C.; Visser, F.C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients with Joint Hypermobility Show Larger Cerebral Blood Flow Reductions during Orthostatic Stress Testing Than Patients without Hypermobility: A Case Control Study. Med. Res. Arch. 2021, 9, 18. [Google Scholar] [CrossRef]
- Beighton, P.; Solomon, L.; Soskolne, C.L. Articular mobility in an African population. Ann. Rheum. Dis. 1973, 32, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.J.; Scammell, B.E.; Batt, M.E.; Palmer, D. Development and validation of self-reported line drawings of the modified Beighton score for the assessment of generalised joint hypermobility. BMC Med Res. Methodol. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Tannemaat, M.R.; van Niekerk, J.; Reijntjes, R.H.; Thijs, R.D.; Sutton, R.; van Dijk, J.G. The semiology of tilt-induced psychogenic pseudosyncope. Neurology 2013, 81, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Heyer, G.L.; Harvey, R.A.; Islam, M.P. Signs of autonomic arousal precede tilt-induced psychogenic nonsyncopal collapse among youth. Epilepsy Behav. 2018, 86, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Rowe, A.A.; Fleisch, S.B.; Paranjape, S.; Arain, A.M.; Nicolson, S.E. Psychogenic Pseudosyncope: Diagnosis and Management. Auton. Neurosci. 2014, 184, 66–72. [Google Scholar] [CrossRef]
- Tannemaat, M.R.; Thijs, R.D.; Van Dijk, J.G. Managing psychogenic pseudosyncope: Facts and experiences. Cardiol. J. 2014, 21, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Blad, H.; Lamberts, R.J.; Van Dijk, J.G.; Thijs, R.D. Tilt–induced vasovagal syncope and psychogenic pseudosyncope: Overlapping clinical entities. Neurology 2015, 85, 2006–2010. [Google Scholar] [CrossRef]
- Heyer, G.L.; Harvey, R.A.; Islam, M.P. Comparison of Specific Fainting Characteristics Between Youth With Tilt-Induced Psychogenic Nonsyncopal Collapse Versus Reflex Syncope. Am. J. Cardiol. 2017, 119, 1116–1120. [Google Scholar] [CrossRef]
- Sheldon, R.; Rose, S.; Connolly, S.; Ritchie, D.; Koshman, M.-L.; Frenneaux, M. Diagnostic criteria for vasovagal syncope based on a quantitative history. Eur. Heart J. 2005, 27, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.; Rose, S.; Ritchie, D.; Connolly, S.J.; Koshman, M.-L.; Lee, M.A.; Frenneaux, M.; Fisher, M.; Murphy, W. Historical criteria that distinguish syncope from seizures. J. Am. Coll. Cardiol. 2002, 40, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Van Campen, L.M.C.; Rowe, P.C.; Visser, F.C. Cerebral blood flow remains reduced after tilt testing in myalgic encephalomyelitis/chronic fatigue syndrome patients. Clin. Neurophysiol. Pract. 2021, 6, 245–255. [Google Scholar] [CrossRef]
- Van Campen, L.M.C.; Rowe, P.C.; Visser, F.C. Reductions in Cerebral Blood Flow Can Be Provoked by Sitting in Severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients. Healthcare 2020, 8, 394. [Google Scholar] [CrossRef]
- Van Campen, L.M.C.; Rowe, P.C.; Visser, F.C. Cerebral Blood Flow Is Reduced in Severe Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients During Mild Orthostatic Stress Testing: An Exploratory Study at 20 Degrees of Head-Up Tilt Testing. Healthcare 2020, 8, 169. [Google Scholar] [CrossRef]
- Van Campen, L.M.C.; Verheugt, F.W.A.; Visser, F.C. Cerebral blood flow changes during tilt table testing in healthy volunteers, as assessed by Doppler imaging of the carotid and vertebral arteries. Clin. Neurophysiol. Pract. 2018, 3, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ogoh, S.; Hirasawa, A.; Oue, A.; Sadamoto, T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J. Physiol. 2011, 589, 2847–2856. [Google Scholar] [CrossRef]
- Van Campen, L.M.C.; Visser, F.C. Validation of Stroke volume measured with suprasternal aortic Doppler imaging: Comparison to transthoracic Stroke Volume measurements. J. Thromb. Circ. 2018, 2, 1–5. [Google Scholar]
- Van Campen, L.M.C.; Visser, F.C.; de Cock, C.C.; Vos, H.S.; Kamp, O.; Visser, C.A. Comparison of the haemodynamics of different pacing sites in patients undergoing resynchronisation treatment: Need for individualisation of lead localisation. Heart 2006, 92, 1795–1800. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.; Venet, T.; Schiller, N.B.; Sebastian, A.; Foster, E. Measurement of aortic blood flow by Doppler echocardiography: Temporal, technician, and reader variability in normal subjects and the application of generalizability theory in clinical research. J. Am. Soc. Echocardiogr. 1995, 8, 647–653. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011, 161, 46–48. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Grubb, B.P., 2nd; Olshansky, B.; Shen, W.K.; Calkins, H.; Brignole, M. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015, 12, e41–e63. [Google Scholar] [CrossRef] [Green Version]
- Fedorowski, A.; Burri, P.; Melander, O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J. Hypertens. 2009, 27, 976–982. [Google Scholar] [CrossRef]
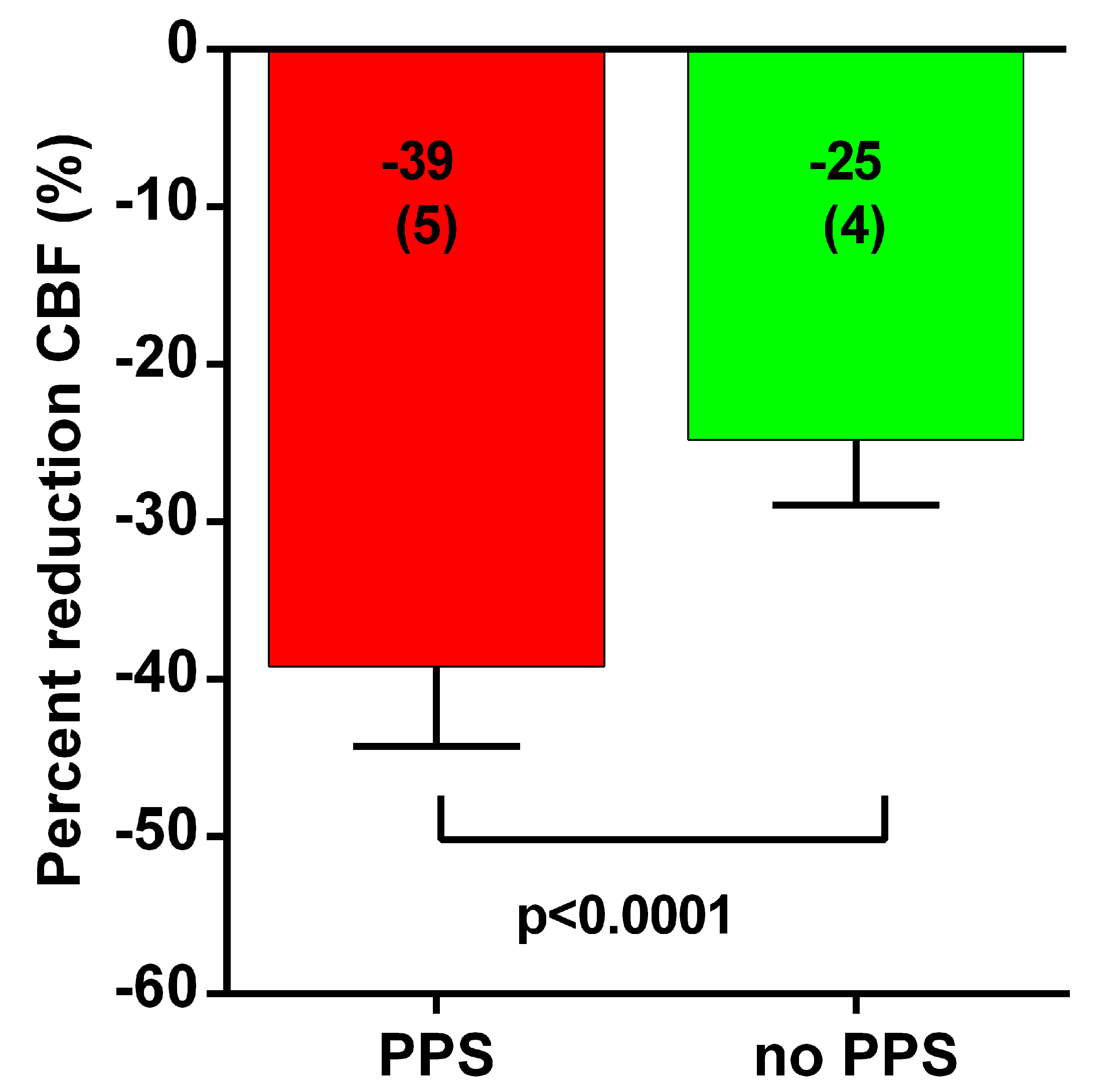
| Cases PPS | Controls No PPS | p-Value | |
|---|---|---|---|
| Heart rate supine (bpm) | 80 (14) | 71 (10) | ns |
| Heart rate upright (bpm) | 111 (22) | 101 (21) | ns |
| SBP supine (mmHg) | 134 (17) | 133 (11) | ns |
| SBP upright (mmHg) | 126 (20) | 127 (20) | ns |
| DBP supine (mmHg) | 81 (13) | 80 (11) | ns |
| DBP upright (mmHg) | 87 (14) | 85 (14) | ns |
| CO supine (L/min) | 4.96 (0.87) | 4.63 (0.79) | ns |
| CO upright (L/min) | 3.68 (1.08) | 3.59 (0.67) | ns |
| CBF supine (mL) | 616 (76) | 601 (118) | ns |
| CBF upright (mL) | 374 (52) | 451 (86) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Campen, C.M.C.; Visser, F.C. Psychogenic Pseudosyncope: Real or Imaginary? Results from a Case-Control Study in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Patients. Medicina 2022, 58, 98. https://doi.org/10.3390/medicina58010098
van Campen CMC, Visser FC. Psychogenic Pseudosyncope: Real or Imaginary? Results from a Case-Control Study in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Patients. Medicina. 2022; 58(1):98. https://doi.org/10.3390/medicina58010098
Chicago/Turabian Stylevan Campen, C. (Linda) M. C., and Frans C. Visser. 2022. "Psychogenic Pseudosyncope: Real or Imaginary? Results from a Case-Control Study in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Patients" Medicina 58, no. 1: 98. https://doi.org/10.3390/medicina58010098
APA Stylevan Campen, C. M. C., & Visser, F. C. (2022). Psychogenic Pseudosyncope: Real or Imaginary? Results from a Case-Control Study in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Patients. Medicina, 58(1), 98. https://doi.org/10.3390/medicina58010098






