Abstract
Allograft vesicoureteral reflux (VUR) is a leading urological complication of kidney transplantation. Despite the relatively high incidence, there is a lack of consensus regarding VUR risk factors, impact on renal function, and management. Dialysis vintage and atrophic bladder have been recognized as the most relevant recipient-related determinants of post-transplant VUR, whilst possible relationships with sex, age, and ureteral implantation technique remain debated. Clinical manifestations vary from an asymptomatic condition to persistent or recurrent urinary tract infections (UTIs). Voiding cystourethrography is widely accepted as the gold standard diagnostic modality, and the reflux is generally graded following the International Reflux Study Committee Scale. Long-term transplant outcomes of recipients with asymptomatic grade I-III VUR are yet to be clarified. On the contrary, available data suggest that symptomatic grade IV-V VUR may lead to progressive allograft dysfunction and premature transplant loss. Therapeutic options include watchful waiting, prolonged antibiotic suppression, sub-mucosal endoscopic injection of dextranomer/hyaluronic acid copolymer at the site of the ureteral anastomosis, and surgery. Indication for specific treatments depends on recipient’s characteristics (age, frailty, compliance with antibiotics), renal function (serum creatinine concentration < 2.5 vs. ≥ 2.5 mg/dL), severity of UTIs, and VUR grading (grade I-III vs. IV-V). Current evidence supporting surgical referral over more conservative strategies is weak. Therefore, a tailored approach should be preferred. Properly designed studies, with adequate sample size and follow-up, are warranted to clarify those unresolved issues.
1. Introduction
Vesicoureteral reflux (VUR) represents one of the most frequently observed urological complications of kidney transplantation (KT) [1,2,3]. It is defined as an abnormal flow of urine backward from the bladder to the ureter or, in extreme cases, up to the renal pelvis. In the general population, VUR can be either congenital (primary) or acquired (secondary), following blockage or failure of the bladder musculature, as well as dysfunction of the nerves controlling bladder emptying [4]. Clinical features range from an asymptomatic condition to end-stage renal disease (ESRD) and appear as directly related to the severity of the reflux and the occurrence of urinary tract infections (UTIs) [5,6,7]. The technique adopted for ureteral implantation and bladder function, at the time of transplant, certainly are key factors in the development of post-operative VUR. The correlation between post-transplant VUR and increased risk of UTIs is undisputed. Nonetheless, the transplant community has yet to reach an agreement on optimal pre-emptive strategy, clinical relevance, management, and impact on long-term allograft function and survival [8].
2. Literature Research
We performed systematic research to identify epidemiology, clinical presentation, diagnosis, staging, prognosis, treatment options, and follow-up strategies for VUR in adult KT recipients. We used the electronic databases PubMed and Embase. No time limits were applied, but we included studies published before 30 September 2021. We chose the following MeSH terms for the research through PubMed: “kidney”, “transplantation”, and “vesicoureteral reflux”. We used the following string to search through Embase: (‘transplantation’/exp AND ‘kidney’/exp AND ‘vesicoureteral reflux’/exp). Inclusion criteria were: all kinds of articles, including conference papers, but excluding case reports, all languages. Exclusion criteria were: species (non-human), age (paediatric recipients), and type of article (case reports). This topic has rarely been met with standard screening and reporting criteria, which prevented us from performing meta-analyses. As a consequence, we also excluded 251 articles because they were not amenable to a meaningful qualitative analysis. The selection process is displayed in Figure 1.
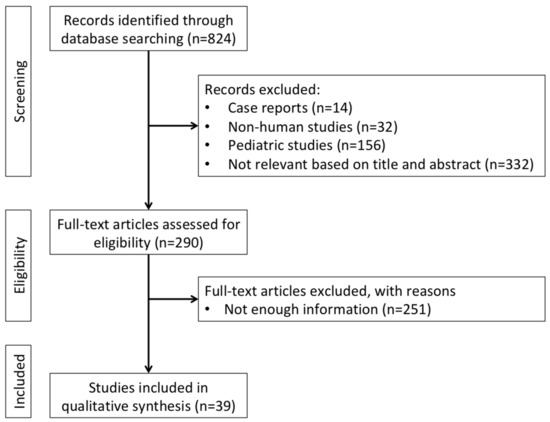
Figure 1.
Flowchart of literature research and study selection.
3. Epidemiology, Etiology, and Risk Factors for Post-Transplant VUR
The incidence of VUR after KT extends from 0.5 to 86%. This remarkably wide range depends on the highly variable criteria that are employed to screen KT recipients for VUR and on the reporting methodology [9,10].
Over the years, several factors have been recognized as increasing the risk of post-transplant VUR. They can be sorted in two main categories: modifiable and unmodifiable risk factors. Unmodifiable risk factors include recipient-related characteristics, such as sex, urinary tract abnormalities, or neurological disorders, whereas modifiable risk factors are surgical technique and surgeon’s expertise (Table 1) [11].

Table 1.
Risk factors for post-transplant vesicoureteral reflux.
The association between sex and urological complications has been the subject of multiple investigations. Most literature suggests that females may be more prone to allograft VUR and UTIs [11,12,13,14]. However, in a study specifically designed to assess the effect of sex on VUR, Farr et al. observed that, on multivariate analysis, no sex-specific differences could be detected [15].
Further independent risk factors for post-transplant VUR are recipient age, non-Caucasian ethnicity, hypertension, type 2 diabetes mellitus, lower urinary tract abnormalities, and continuous or intermittent bladder catheterization [14]. Among the others, bladder conformation is probably the most important determinant. Indeed, recipients who have been on renal replacement therapy (RRT) for years, very often exhibit a hypo-compliant bladder with low capacity, thin walls, and high intra-vesical pressure [16]. Such a condition may represent a technical challenge when performing the ureteral implantation and may increase the risk of peri-operative complications [17]. In a recent study on 408 KT recipients, investigating possible relationships between duration of RRT, bladder capacity, and post-transplant urological complications, it has been observed that the incidence of VUR is significantly higher in dialysis vintage patients with an atrophic bladder (capacity < 50 mL) than those who have a bladder capacity of 50 mL or above, thus raising the hypothesis that atrophic bladder could actually predispose to VUR [18]. Inoue et al. have reported conflicting results [19,20]. In a study published in 2011, the authors found that intra-vesical pressure influenced the prevalence of VUR, which was higher among recipients with small atrophic bladders [19]. Nevertheless, in 2016, the same group showed that there were no statistically significant differences in VUR or other post-transplant urological complication rates between patients with or without a hypo-compliant bladder [20]. Such discrepancy has been arbitrarily ascribed to modifications in the surgical technique. In particular, it has been postulated that the difference in VUR rates was related to the adoption of a 3-mm sub-mucosal ureteral tunnel during the second study, which minimized the occurrence of VUR [20].
Multiple options for ureteral implantation are currently available. When the Lich–Grégoir technique is chosen, a single cystostomy is performed and the anastomosis is carried out between the distal ureter and the bladder’s mucosa; the detrusor muscle is oversewn to provide an anti-reflux tunnel [21,22]. The Politano–Leadbetter technique requires two cystostomies. The transplant ureter is introduced into the bladder through the first cystostomy, tunnelled for several millimetres, and eventually anastomosed to the bladder’s mucosa through the second cystostomy; the detrusor muscle is approximated to create an anti-reflux mechanism [23]. In the full-thickness technique, the ureter is anastomosed to the bladder’s wall, with full-thickness stitches, without tunnelling [24]. In case the U-stitch technique is preferred, one or two stitches are thrown at the distal tip of the ureter, brought through the bladder’s wall, and tied [25]. If the recipient has no ureteral abnormalities, an uretero-ureteral anastomosis between the allograft ureter and the native ureter can be also considered, ensuring a natural anti-reflux mechanism [14]. The Lich–Grégoir and the Politano-Leadbetter are the most frequently adopted techniques. The vast majority of transplant surgeons seem to favour the use of a relatively short ureter and the construction of a large uretero-cystostomy over a tunnelled reimplant, in an effort to reduce the likelihood of ureteral strictures [26]. However, while it is clear and intuitive that operative technique is the main cause of VUR, no general consensus exists on which type of ureteral implantation should be performed during KT. As pointed out by Duty et al., it is plausible that the occurrence and severity of the reflux may be influenced by the particular approach chosen for the ureteral anastomosis [26]. A systematic review and meta-analysis has compared the Lich–Grégoir vs. the Politano–Leadbetter and the Lich–Grégoir vs. the U-stich techniques. Remarkably, the Lich–Grégoir uretero-vesical anastomosis has resulted in fewer post-operative urological complications, including VUR [27]. Regardless of the reconstruction technique, it has become common to insert self-retaining vesicoureteral stents during the procedure. While this practice has proved successful in reducing urinary leaks, indwelling stents can increase the rate of UTIs [9]. What is less clear is whether a risk of VUR, although unlikely, remains after their removal.
The possible impact of surgical expertise on the development of post-transplant VUR has also been evaluated with mixed results. Cash et al. have investigated the effect of surgeons’ experience on overall surgical complication rates after KT. In their study, no differences were observed between experienced and inexperienced surgeons [28]. On the contrary, a multivariate analysis by Farr et al. has shown that surgical experience is the most significant predictor of post-transplant VUR, even after multiple adjustments for potential confounders and sex-specific urological complications [15].
4. Clinical Presentation, Diagnosis, and Grading of Post-Transplant VUR
The main complication of VUR, after KT, is the development of UTIs. Accordingly, the most frequently observed clinical manifestations are dysuria, strangury, urinary frequency, urinary urgency, and fever. Post-transplant UTIs are classified into one of the following categories: asymptomatic bacteriuria, lower UTI, acute graft pyelonephritis (AGPN), and urosepsis. Asymptomatic bacteriuria is defined as the isolation of a bacterial strain with no symptoms of lower or upper UTI, including leukocyturia. Lower UTI involves bacteriuria associated with urinary symptoms (dysuria, urinary frequency, or urinary urgency) and/or mild fever (<38 °C), in the absence of the criteria for AGPN. AGPN requires significant bacteriuria and high fever (≥38 °C) and may be associated with allograft tenderness and acute transplant dysfunction. The diagnosis of urosepsis is made when the same bacterial strain can be isolated in simultaneous blood and urine cultures. UTIs can be also classified as new infections, relapses, or re-infections. Relapse is defined as the isolation of the same microorganism, which caused the first infection, in urine cultures obtained two or more weeks after completion of antibiotic treatment; re-infections are UTIs caused by a new agent that is different from the one isolated during the course of the previous infection [29].
In the vast majority of patients, the presence of allograft VUR can be easily detected before clinical onset. Even though the necessity and timing of VUR screening after KT remain debated, Hotta et al. have demonstrated that a voiding cystourethrography (VCUG), performed in the very early post-transplant phase (namely, at the time of bladder catheter removal), is a safe and feasible option [18]. Current policy is to wait for persistent or recurrent UTIs before proceeding with a diagnostic work-up, which may include a pelvic Doppler ultrasound (US) scan, a contrast-enhanced abdomen computed tomography (CT) scan, or a VCUG.
The gold standard imaging modality for both diagnosis and grading of VUR after KT is certainly a VCUG. This fluoroscopic study is obtained by introducing an iodinate contrast medium into the bladder via a temporary catheter, and it can easily detect VUR, as well as other bladder or urethral abnormalities. An US scan is often performed for prompt evaluation of the allograft. In asymptomatic recipients without hydronephrosis or impaired bladder emptying, no further investigation is generally carried out. In case of symptomatic UTIs with allograft dysfunction, a contrast-enhanced CT scan or a VCUG may be required [18,30]. According to the International Reflux Study Committee Scale, post-transplant VUR can be graded as: Grade I, reflux of urine limited to the ureter; Grade II, reflux of urine into the ureter, renal pelvis, and calyces without dilation; Grade III, reflux of urine that causes a mild-to-moderate dilation of the ureter, renal pelvis, and calyces with minimal blunting of the fornices; Grade IV, moderate ureteral tortuosity and dilation of renal pelvis and calyces; Grade V, gross dilation of the ureter, renal pelvis, and calyces, with severe swelling and ureteral twisting (Table 2) [31].

Table 2.
Diagnosis and management of post-transplant vesicoureteral reflux.
5. Management of Post-Transplant VUR
When and how to treat a post-transplant VUR have yet to be clarified (Table 2).
The clinical management of VUR occurring in a kidney allograft depends on the grade of the reflux and, most importantly, on the severity and frequency of UTIs. Briefly, the following options are available: wait-and-see strategy [12], antibiotics administration [26], endoscopic injection of dextranomer/hyaluronic acid copolymer into the ureteral implantation site [32], pyelo-ureterostomy between the pelvis of the allograft and the native ureter [33], or ureteral re-implantation [34]. In case of asymptomatic VUR, the most reasonable option is to leave the reflux untreated. For symptomatic low-to-moderate VUR, endoscopic copolymer injection can be considered, in institutions with adequate expertise. The procedure was first described in 1995, and it is routinely performed under local anaesthesia or sedation. The copolymer is cystoscopically injected into the sub-mucosal layer of the bladder, at the site of the ureteral anastomosis, to expand the tunnel surrounding the ureter and, therefore, reduce the reflux. In the original study, the reflux was resolved in 58% of patients after the first injection, while this rate increased to 79% after two injections [32]. These encouraging results have been confirmed by other studies, reporting primary treatment success rates as high as 63% in both living- and deceased-donor KT [35]. In patients with symptomatic moderate-to-high grade VUR, clinical management is based on a variety of factors. Whang et al. recommend a tailored approach, considering allograft function, frequency of recurrent UTIs, compliance with antibiotic treatment, age, and overall health status of the recipient [12]. According to these authors, the degree of the reflux should not be regarded as the major determinant of the need for reconstructive surgery. In patients with a serum creatinine concentration ≥ 2.5 mg/dL, antibiotic suppression without planned end date should be preferred because allograft function is already compromised. Similarly, for elderly or frail recipients, as well as those refusing surgery, prolonged antibiotic administration is advocated. In all other cases, patients should be referred for surgical evaluation. The choice of the surgical procedure primarily depends on the availability of a non-refluxing ipsilateral ureter [36]. Whenever possible, an uretero-ureterostomy between the allograft ureter and the native ureter represents the best option. In fact, using the native ureter provides a better anti-reflux mechanism and easier access, should endoscopic evaluation or treatment be necessary in the future [33]. The anastomosis can be performed in either an end-to-end or an end-to-side fashion, depending on the length and size of the transplant ureter. Urological stents are usually inserted in both ureters before the operation to reduce the risk of unintentional damage during dissection. When a suitable native ureter is not available, a new uretero-cystostomy is performed using the transplant ureteral stump, possibly adopting the Politano–Leadbetter technique [12]. It is also worth mentioning the technique proposed by Turunça et al., which consists of an extra-vesical seromuscular tunnel lengthening [37]. This procedure aims to increase the length of the seromuscular tunnel to more than 3 cm to cover the distal segment of the transplanted ureter. Comparison between tunnel lengthening, uretero-ureterostomy, and pyelo-ureteral anastomosis have shown lower surgical complication and recurrent UTIs rates, better allograft function, shorter operative times and hospital stays following tunnel lengthening [37]. Overall, excellent results have been described after reconstructive surgery, regardless of the specific technique chosen for ureteral reimplantation. Patil et al. have reported that 95% of the patients who had undergone surgery were relieved from symptomatic UTIs and freed from antibiotic suppression [36]. However, considering the risk associated with general anaesthesia and surgery, some authors suggest a trial of prophylactic antibiotics in all patients with mild-to-moderate symptoms and Grade I-III reflux [26]. While the role of prophylaxis against Pneumocystis jirovecii pneumonia with trimethoprim-sulfamethoxazole is not an option for the treatment of VUR itself, it has been shown that prophylactic antibiotics reduce the frequency of bacterial infections in KT recipients, including UTIs. Consequently, the last KDIGO guidelines recommended prophylactic trimethoprim–sulfamethoxazole for six-twelve months after transplant [8]. Since UTIs are a significant source of morbidity in recipients with VUR, it can be argued that indefinite prophylaxis with trimethoprim-sulfamethoxazole might be of some use in this category, though the available evidence is poor.
As a last suggestion, biofeedback therapy might constitute a promising approach to VUR, at least for recipients with proven dysfunctional voiding. The role of biofeedback, intended as the set of practices meant to establish appropriate bladder control and micturition, has not been explored in KT, and this lack of evidence makes it worth studying biofeedback treatments in prospective trials.
6. Impact of Post-Transplant VUR on Allograft Function and Survival
The impact of VUR on transplant-related outcomes is still unclear [10,38,39]. Margreiter et al. performed one of the largest studies assessing the association between VUR and allograft survival in both living- and deceased-donor KT. They used a standardized per-protocol VCUG to evaluate 646 consecutive recipients before hospital discharge. The prevalence of VUR was 41%. One year after transplant, the estimated glomerular filtration rate was significantly lower in patients with VUR than controls. However, such a difference was not confirmed at three and five years of follow-up. Sorting patients according to the severity of the reflux did not affect results, as recipient and allograft survival, as well as cell-mediated and antibody-mediated rejection rates remained comparable [30]. In a similarly designed study with a smaller sample size, our group also demonstrated that VUR did not influence long-term allograft function and survival. However, this conclusion is limited to Grade I-III VUR, as in the population included there were no patients with Grade IV-V VUR [40]. Coulthard et al. have used the dimercaptosuccinic acid (DMSA) scan to evaluate allograft morphology, function, and scarring in recipients with or without VUR. In up to 40% of the patients with documented VUR and UTIs, a specific pattern of photon-deficient areas could be observed. Such peculiar scarring was associated with irreversible loss of function in half of the subjects [41]. Obha et al. reported long-term allograft dysfunction in KT recipients with VUR and AGPN [42]. The detailed information, arising from the systematic review of the literature, is fully reported in Table 3 and Table 4.

Table 3.
Epidemiology of kidney transplant recipients with vesicoureteral reflux.

Table 4.
Clinical features of kidney transplant recipients with vesicoureteral reflux.
7. Conclusions
VUR represents a leading urological complication of KT. Atrophic bladder is the most relevant predisposing factor, and it is predominantly related to pre-existing urological disorders or dialysis vintage. The surgical technique used for ureteral implantation is considered as another possible determinant, but supporting evidence is scarce. Post-transplant VUR should be graded according to the International Reflux Study Committee Scale. The application of a rigorous and consistent grading system is recommended for proper data collection and analysis. Clinical presentation is extremely variable. The vast majority of recipients with VUR is asymptomatic. Main complaints are related to the development of persistent or recurrent UTIs. To date, there are no standardized screening protocols. Nevertheless, VCUG is widely accepted as the gold standard diagnostic modality. Clinical guidelines for the management of VUR after KT are lacking. Reasonably, asymptomatic patients can be followed-up with a wait-and-see strategy. Symptomatic subjects, with complex comorbidities or irreversible allograft dysfunction, as well as those refusing more invasive options, are preferably treated with prolonged antibiotic suppression. For symptomatic recipients, with preserved transplant function and low-to-moderate VUR, endoscopic copolymer injection may be considered. In case of endoscopic treatment failure or severe VUR, patients should be offered surgical reconstruction.
Over the last two decades, a number of studies have been performed to evaluate incidence, risk factors, and consequences of VUR after KT. Several diagnostic protocols and treatment strategies have also been proposed. Despite this great effort, evidence remains weak in any relevant aspect of the topic. In particular, it is of paramount importance to clarify whether VUR may jeopardize long-term allograft function and survival. Future research will have to minimize the multiple bias affecting previous studies. Timely identification of recipients with VUR, at higher risk of UTIs, will likely improve outcomes, as recurrent UTIs represent the leading complication of VUR and a well-recognized risk factor for post-transplant morbidity and mortality.
Author Contributions
Conceptualization, E.F.; independent literature research, A.B., S.G., R.D., M.P., C.M.A. and C.P.; writing—original draft preparation, A.B., S.I., C.M.A. and R.D.; writing—review and editing, S.I., R.D., M.P. and C.P.; visualization, S.I., E.F. and M.F.; supervision, C.M.A., M.F. and E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Jean-Sébastien Marre for his kind linguistic revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Whang, M.; Yballe, M.; Geffner, S.; Fletcher, H.S.; Palekar, S.; Mulgaonkar, S. Urologic complications in more than 2500 kidney transplantations performed at the Saint Barnabas healthcare system. Transplant. Proc. 2011, 43, 1619. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.L.; Zhang, K.Q.; Li, Q.S.; Jin, F.S.; Zhu, F.Q.; Huo, W.Q. Urological Complications in 1223 Kidney Transplantations. Urol. Int. 2009, 83, 337–341. [Google Scholar] [CrossRef]
- Zavos, G.; Pappas, P.; Karatzas, T.; Karidis, N.; Bokos, J.; Stravodimos, K.; Theodoropoulou, E.; Boletis, J.; Kostakis, A. Urological Complications: Analysis and Management of 1525 Consecutive Renal Transplantations. Transplant. Proc. 2008, 40, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Fletcher, J.T.; Alexander, S.I.; Craig, J.C. Vesicoureteral Reflux. J. Am. Soc. Nephrol. 2008, 19, 847–862. [Google Scholar] [CrossRef]
- Tekgül, S.; Riedmiller, H.; Hoebeke, P.; Kočvara, R.; Nijman, R.J.; Radmayr, C.; Stein, R.; Dogan, H.S. EAU Guidelines on Vesicoureteral Reflux in Children. Eur. Urol. 2012, 62, 534–542. [Google Scholar] [CrossRef]
- Mattoo, T.K.; Chesney, R.W.; Greenfield, S.P.; Hoberman, A.; Keren, R.; Mathews, R.; Gravens-Mueller, L.; Ivanova, A.; Carpenter, M.A.; Moxey-Mims, M.; et al. RIVUR Trial Investigators. Renal Scarring in the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR). Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 54–61. [Google Scholar] [CrossRef]
- Gołębiewska, J.; Dębska-Ślizień, A.; Komarnicka, J.; Samet, A.; Rutkowski, B. Urinary tract infections in renal trans-plant recipients. Transplant. Proc. 2011, 43, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. 3), S1–S155. [Google Scholar]
- Ostrowski, M.; Włodarczyk, Z.; Wesołowski, T.; Gracz, H.; Sluzar, T.; Sieńko, J.; Lubikowski, J.; Bohatyrewicz, R. Influence of ureterovesical anastomosis technique on the incidence of vesicoureteral reflux in renal transplant recipients. Ann. Transplant. 1999, 4, 54–58. [Google Scholar]
- Mastrosimone, S.; Pignata, G.; Maresca, M.C.; Calconi, G.; Rabassini, A.; Butini, R.; Fandella, A.; Di Falco, G.; Chiara, G.; Caldato, C. Clinical significance of vesicoureteral reflux after kidney transplantation. Clin. Nephrol. 1993, 40, 38–45. [Google Scholar]
- Sui, W.; Lipsky, M.J.; Matulay, J.T.; Robins, D.J.; Onyeji, I.C.; James, M.B.; Theofanides, M.C.; Wenske, S. Timing and Predictors of Early Urologic and Infectious Complications After Renal Transplant: An Analysis of a New York Statewide Database. Exp. Clin. Transplant. 2018, 16, 665–670. [Google Scholar]
- Whang, M.; Benson, M.; Salama, G.; Geffner, S.; Sun, H.; Aitchison, S.; Mulgaonkar, S. Urologic Complications in 4000 Kidney Transplants Performed at the Saint Barnabas Health Care System. Transplant. Proc. 2020, 52, 186–190. [Google Scholar] [CrossRef]
- Gołębiewska, J.; Dębska-Ślizień, A.; Zadrożny, D.; Rutkowski, B. Acute Graft Pyelonephritis During the First Year After Renal Transplantation. Transplant. Proc. 2014, 46, 2743–2747. [Google Scholar] [CrossRef]
- Dinckan, A.; Aliosmanoglu, I.; Kocak, H.; Gunseren, F.; Mesci, A.; Ertug, Z.; Yucel, S.; Suleymanlar, G.; Gurkan, A. Surgical correction of vesico-ureteric reflux for recurrent febrile urinary tract infections after kidney transplantation. BJU Int. 2013, 112, E366. [Google Scholar] [CrossRef]
- Farr, A.; Györi, G.; Mühlbacher, F.; Husslein, P.; Böhmig, G.A.; Margreiter, M. Gender has no influence on VUR rates after renal transplantation. Transpl. Int. 2014, 27, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Lee, M.-C.; Kuo, H.-C. Reduction of cystometric bladder capacity and bladder compliance with time in patients with end-stage renal disease. J. Formos. Med. Assoc. 2012, 111, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.P.; Flechner, S.M.; Modlin, C.S.; Wyner, L.M.; Novick, A.C. Transplantation into the long-term defunctionalized bladder. J. Urol. 1996, 156, 885–888. [Google Scholar] [CrossRef]
- Hotta, K.; Miura, M.; Wada, Y.; Fukuzawa, N.; Iwami, D.; Sasaki, H.; Seki, T.; Harada, H. Atrophic bladder in long-term dialysis patients increases the risk for urological complications after kidney transplantation. Int. J. Urol. 2017, 24, 314–319. [Google Scholar] [CrossRef]
- Inoue, T.; Satoh, S.; Saito, M.; Numakura, K.; Tsuruta, H.; Obara, T.; Narita, S.; Horikawa, Y.; Tsuchiya, N.; Habuchi, T. Correlations Between Pretransplant Dialysis Duration, Bladder Capacity, and Prevalence of Vesicoureteral Reflux to the Graft. Transplant 2011, 92, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Satoh, S.; Obara, T.; Saito, M.; Numakura, K.; Narita, S.; Tsuchiya, N.; Habuchi, T. Cystometric evaluation of recovery in hypocompliant defunctionalized bladder as a result of long-term dialysis after kidney transplantation. Int. J. Urol. 2016, 23, 694–700. [Google Scholar] [CrossRef]
- Gregoir, W. Congenital vesico-ureteral reflux. Acta Urol. Belg. 1962, 30, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Lich, R., Jr.; Howerton, L.W.; Davis, L.A. Childhood urosepsis. J. Ky Med. Assoc. 1961, 59, 1177–1179. [Google Scholar]
- Politano, V.A.; Leadbetter, W.F. An operative technique for the correction of vesicoureteral reflux. J. Urol. 1958, 79, 932–941. [Google Scholar] [CrossRef]
- Kayler, L.; Kang, D.; Molmenti, E.; Howard, R. Kidney transplant ureteroneocystostomy techniques and complications: Review of the literature. Transplant. Proc. 2010, 42, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Shanfield, I. New experimental methods for implantation of the ureter in bladder and conduit. Transplant. Proc. 1972, 4, 637–638. [Google Scholar] [PubMed]
- Duty, B.D.; Barry, J.M. Diagnosis and management of ureteral complications following renal transplantation. Asian J. Urol. 2015, 2, 202–207. [Google Scholar] [CrossRef][Green Version]
- Alberts, V.P.; Idu, M.M.; Legemate, D.A.; Pes, M.P.L.; Minnee, R.C. Ureterovesical anastomotic techniques for kidney transplantation: A systematic review and meta-analysis. Transpl. Int. 2014, 27, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.; Slowinski, T.; Buechler, A.; Grimm, A.; Friedersdorff, F.; Schmidt, D.; Miller, K.; Giessing, M.; Fuller, T.F. Impact of surgeon experience on complication rates and functional outcomes of 484 deceased donor renal transplants: A single-centre retrospective study. BJU Int. 2012, 110, E368–E373. [Google Scholar] [CrossRef]
- Gołębiewska, J.E.; Dębska-Ślizień, A.; Rutkowski, B. Urinary tract infections during the first year after renal transplantation: One center’s experience and a review of the literature. Clin. Transplant. 2014, 28, 1263–1270. [Google Scholar] [CrossRef]
- Margreiter, M.; Györi, G.P.; Böhmig, G.A.; Trubel, S.; Mühlbacher, F.; Steininger, R. Value of routine voiding cystourethrography after renal transplantation. Am. J. Transplant. 2013, 13, 130–135. [Google Scholar] [CrossRef]
- Lebowitz, R.L.; Olbing, H.; Parkkulainen, K.V.; Smellie, J.M.; Tamminen-Möbius, T.E. International system of radio-graphic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr. Radiol. 1985, 15, 105–109. [Google Scholar] [CrossRef]
- Stenberg, A.; Lackgren, G. A New Bioimplant for the Endoscopic Treatment of Vesicoureteral Reflux: Experimental and Short-term Clinical Results. J. Urol. 1995, 154, 800–803. [Google Scholar] [CrossRef]
- Yang, K.K.; Moinzadeh, A.; Sorcini, A. Minimally-Invasive Ureteral Reconstruction for Ureteral Complications of Kidney Transplants. Urology 2019, 126, 227–231. [Google Scholar] [CrossRef]
- Di Carlo, H.N.; Darras, F.S. Urologic considerations and complications in kidney transplant recipients. Adv. Chronic Kidney Dis. 2015, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, K.; Choi, S.; Bae, W.; Hong, S.; Lee, J.; Kim, J.; Kim, S.; Chung, B.; Yang, C.; et al. Ureteral Complications in Kidney Transplantation: Analysis and Management of 853 Consecutive Laparoscopic Living-Donor Nephrectomies in a Single Center. Transplant. Proc. 2016, 48, 2684–2688. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Geffner, S.; Sun, H.; Whang, M. Surgical Treatment of Vesicoureteral Reflux in Kidney Transplant Patients with Symptomatic Urinary Tract Infection: A Single Institution Review of 123 Patients. Clin. Surg. 2016, 1, 1160. [Google Scholar]
- Turunç, V.; Eroğlu, A.; Tabandeh, B.; Erol, A. Comparison of Surgical Correction Techniques for Post-Renal Transplantation Vesicoureteral Reflux. Transplant. Proc. 2017, 49, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.A.; McRoberts, J.W.; Curtis, J.J.; Luke, R.G. Controversy in Renal Transplantation: Antireflux Versus Non-Antireflux Ureteroneocystostomy. J. Urol. 1979, 121, 156–158. [Google Scholar] [CrossRef]
- Jung, G.O.; Chun, J.M.; Park, J.B.; Choi, G.S.; Kwon, C.H.; Joh, J.W.; Lee, S.K.; Kim, S.J. Clinical significance of posttransplan-tation vesicoureteral reflux during short-term period after kidney transplantation. Transplant. Proc. 2008, 40, 2339–2341. [Google Scholar] [CrossRef]
- Favi, E.; Spagnoletti, G.; Valentini, A.; Tondolo, V.; Nanni, G.; Citterio, F.; Castagneto, M. Long-Term Clinical Impact of Vesicoureteral Reflux in Kidney Transplantation. Transplant. Proc. 2009, 41, 1218–1220. [Google Scholar] [CrossRef]
- Coulthard, M.G.; Keir, M.J. Reflux Nephropathy in Kidney Transplants, Demonstrated by Dimercaptosuccinic Acid Scanning. Transplantaton 2006, 82, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ohba, K.; Matsuo, M.; Noguchi, M.; Nishikido, M.; Koga, S.; Kanetake, H.; Nazneen, A.; Liu, D.; Razzaque, M.S.; Taguchi, T. Clinicopathological study of vesicoureteral reflux (VUR)-associated pyelonephritis in renal transplantation. Clin. Transplant. 2004, 18 (Suppl. 11), 34–38. [Google Scholar] [CrossRef]
- Mathew, T.H.; Mathews, D.C.; Hobbs, J.B.; Kincaid-Smith, P. Glomerular lesions after renal transplantation. Am. J. Med. 1975, 59, 177–190. [Google Scholar] [CrossRef]
- Kmetec, A.; Buturović-Ponikvar, J.; Kandus, A.; Bren, A.F. The value of renal resistive index for the detection of vesicoureteral reflux in renal transplant recipients. Transplant. Proc. 2001, 33, 3385–3387. [Google Scholar] [CrossRef]
- Praz, V.; Leisinger, H.-J.; Pascual, M.; Jichlinski, P. Urological Complications in Renal Transplantation from Cadaveric Donor Grafts: A Retrospective Analysis of 20 Years. Urol. Int. 2005, 75, 144–149. [Google Scholar] [CrossRef]
- Obara, T.; Satoh, S.; Inoue, T.; Komine, N.; Numakura, K.; Narita, S.; Horikawa, Y.; Tsuchiya, N.; Habuchi, T. Impact of Pretransplant Dialysis Duration, Bladder Capacity and Length of Submucosal Tunnel of Ureteroneocystostomy on the Prevalence of Vesicoureteral Reflux to the Graft. In Meeting Abstracts of the 24th International Congress of the Transplantation Society, Berlin, Germany, 14–19 July 2012. Transplantation 2012, 94, 269. [Google Scholar] [CrossRef]
- Sandhu, K.; Masters, J.; Ehrlich, Y. Ureteropyelostomy using the native ureter for the management of ureteric obstruction or symptomatic reflux following renal transplantation. Urology 2012, 79, 929–932. [Google Scholar] [CrossRef]
- Li Marzi, V.; Del Popolo, G.; Filocamo, M.T.; Milanesi, M.; Cocci, A.; Tosto, A.; Nicita, G. Our experience related to uro-dynamic assessment in renal transplant recipients. In Abstracts from the 37th Annual Congress of the Italian Urodynamic Society, Latina, Italy, 20–22 June 2013. Neurourol. Urodyn. 2013, 32, S1–S58. [Google Scholar]
- Riediger, C.; Müller, M.W.; Bachmann, J.; Novotny, A.; Thorban, S.; Matevossian, E.; Friess, H.; Stangl, M. Native ureteropyelostomy: An effective therapy for urinary tract complications following kidney transplantation. ANZ J. Surg. 2014, 84, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Ghazanfar, A.; Bagul, A.; Salem, A.; Ali, A.; Wadoodi, A.; Heap, S.; Morsy, M. Incidence and outcome of post-transplant urological complications in kidney transplantation after expanding acceptance criteria: Single centre experience. In Abstracts of the 2016 TTS Congress, Hong Kong, China, 18–23 August. Transplantation 2016, 100, S641. [Google Scholar]
- Koçak, T.; Nane, I.; Ander, H.; Ziylan, O.; Oktar, T.; Ozsoy, C. Urological and surgical complications in 789 consecutive living related donor kidney transplantations. In Proceedings of the Abstracts of the 18th Congress of the European Society for Organ Transplantation, Barcelona, Spain, 24–27 September 2017. Urol. Int. 2004, 72, 252–256. [Google Scholar] [CrossRef]
- Gutiérrez-Jiménez, A.A.; Jiménez-López, L.A.; Ricardez-Espinosa, A.A.; Santos-Uscanga, J.P.; Aguilar-Sandoval, E.G.; Vega-Tepos, I.E.; George-Micceli, E. Endourological application of polydimetilsiloxane in patients with symptomatic vesicoureteral reflux in the kidney graft. Actas Urol. Esp. (Engl. Ed.) 2019, 43, 262. [Google Scholar] [CrossRef] [PubMed]
- Di Lascio, G.; Di Pierro, G.B.; Angelini, F.; Cantisani, V.; Drudi, F.M.; Lemma, A.; Cristini, C. Contrast-Enhanced voiding ultrasonography in the evaluation of vesicoureteral reflux: Comparison with voiding cystourethrography and proposal of a new classification system. In Abstracts from the 93rd National Congress of the Italian Society of Urology, Rome, Italy, 17–18 October. Eur. Urol. Open Sci. 2020, 20, S98. [Google Scholar]
- Ladhari, N.; Azzabi, A.; Sahtout, W.; Guedri, Y.; Mrabet, S.; Fradi, A.; Zallema, D.; Abdellatif, A. Nosocomial infections in kidney transplant patients: A single center experience. In Abstracts from the 2021 ISN World Congress of Nephrology, Montreal, Canada, 15–19 April 2021. Kidney Int. Rep. 2021, 6, S341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).