A Lower Level of Post-Vaccinal Antibody Titer against Influenza Virus A H1N1 May Protect Patients with Autoimmune Rheumatic Diseases from Respiratory Viral Infections
Abstract
1. Introduction
Hypothesis
| Variable | Descriptive Statistics 1 | Inferential Statistics | ||||
|---|---|---|---|---|---|---|
| Type | Name | All | SLE | RA | SS | Comparing SLE, RA and SS |
| Continuous | Age | 60.4 (11.2) 2 | 54.5 (10.5) | 65.6 (7.9) | 66.1 (10.5) | |
| Disease duration | 11.6 (5.8) | 13.0 (3.2) | 13.1 (7.7) | 6.5 (4.7) | ||
| Relative disease duration | 0.20 (0.10) | 0.24 (0.07) | 0.20 (0.12) | 0.11 (0.09) | ||
| Binary | Vaccination 5 | 30% | 25% | 46.7% | 18.2% | |
| Vaccination 4 | 30% | 25% | 46.7% | 18.2% | ||
| Vaccination 3 | 36% | 33.3% | 46.7% | 27.3% | ||
| Vaccination 2 | 44% | 50% | 46.7% | 27.3% | ||
| Vaccination 1 | 56% | 54.2% | 73.3% | 36.4% | ||
| Vaccination (main) | 68% | 66.7% | 73.3% | 63.6% | ||
| Gender | 82% | 83.3% | 66.7% | 100% | ||
| Smoking | 22% | 20.8% | 26.7% | 18.2% | ||
| Bronchitis | 20% | 20.8% | 6.7% | 36.4% | ||
| Therapy | 70% | 79.2% | 80% | 36.4% | ||
| Respiratory viral infection | 38% | 41.7% | 26.7% | 45.5% | ||
| Categorical | Titer | 8 3 | 12 | 0 | 8 | |
| Binary | Assumed SPT | 36% | 41.7% | 26.7% | 36.4% | |
| Personal SPT | 48% | 50% | 46.7% | 45.5% | ||
2. Materials and Method
2.1. Sample
2.2. Design and Variables
- Age (in years);
- Disease duration (in years);
- Relative disease duration (defined by disease duration/age);
- Gender (1—female; 0—male);
- Smoking (1—smoker; 0—non-smoker);
- Therapy (1—Methotrexate and/or glucocorticoids; 0—DMARDs and/or glucocorticoids);
- Vaccination (1—vaccinated; 0—non-vaccinated);
- Vaccination 1 (1—vaccinated a year ago; 0—otherwise);
- Vaccination 2 (1—vaccinated two years ago; 0—otherwise);
- Vaccination 3 (1—vaccinated three years ago; 0—otherwise);
- Vaccination 4 (1—vaccinated four years ago; 0—otherwise);
- Vaccination 5 (1—vaccinated five years ago; 0—otherwise);
- Bronchitis (1— had it before the main vaccination; 0— otherwise);
- Respiratory viral infection (RVI) (1—got it after the main vaccination; 0—otherwise);
- Titer (with values 0, 8, 16, 32, …., 1024);
- Assumed sero-protective titer (assumed SPT) (1—Titer ≥ 1:32; 0—otherwise);
- Personal SPT (1—Titer ≥ 1:16; 0—otherwise).
2.3. Treatment and Procedure
2.4. Statistical Analysis
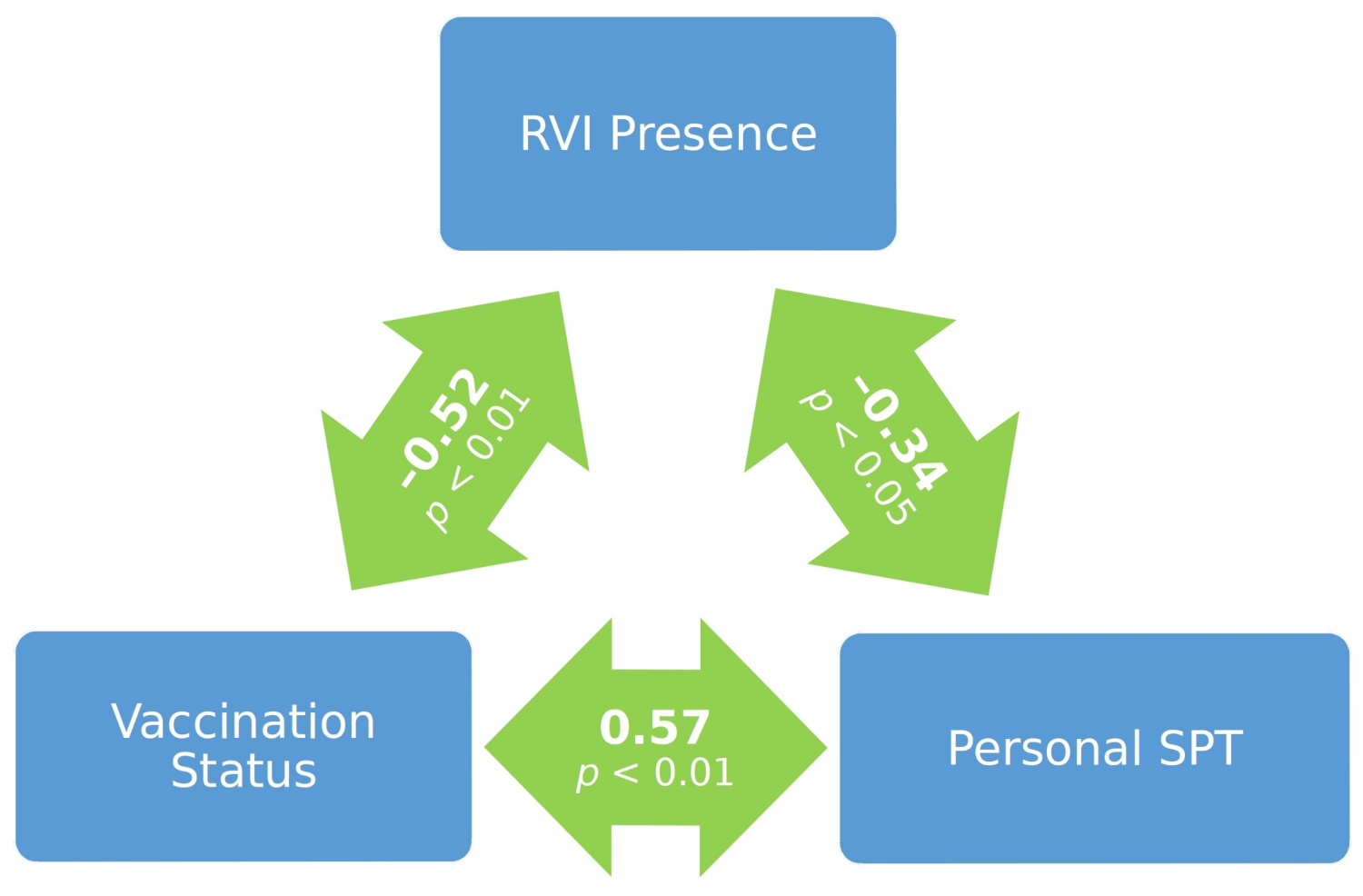
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Franco, M.; Lucchino, B.; Spaziante, M.; Lannuccelli, C.; Valesini, G.; Laiani, G. Lung infections in systemic rheumatic disease: Focus on opportunistic infections. Int. J. Mol. Sci. 2017, 18, 293. [Google Scholar] [CrossRef]
- van Assen, S.; Elkayam, O.; Agmon-Levin, N.; Cervera, R.; Doran, M.F.; Dougados, M.; Emery, P.; Geborek, P.; Ioannidis, J.P.A.; Jayne, D.R.W.; et al. Vaccination in adult patients with auto-immune inflammatory rheumatic diseases: A systematic literature review for the European League Against Rheumatism evidence-based recommendations for vaccination in adult patients with auto-immune inflammatory rheumatic diseases. Autoimmun. Rev. 2011, 10, 341–352. [Google Scholar] [CrossRef]
- Nakafero, G.; Grainge, M.J.; Myles, P.R.; Mallen, C.D.; Zhang, W.; Doherty, M.; Nguyen-Van-Tam, J.S.; Abhishek, A. Predictors and temporal trend of flu vaccination in auto-immune rheumatic diseases in the UK: A nationwide prospective cohort study. Rheumatology 2018, 57, 1726–1734. [Google Scholar] [CrossRef]
- Papadopoulou, D.; Sipsas, N.V. Comparison of national clinical practice guidelines and recommendations on vaccination of adult patients with autoimmune rheumatic diseases. Rheumatol. Int. 2014, 34, 151–163. [Google Scholar] [CrossRef]
- Feng, J.; Gulati, U.; Zhang, X.; Keitel, W.A.; Thompson, D.M.; James, J.A.; Thompson, L.F.; Air, G.M. Antibody quantity versus quality after influenza vaccination. Vaccine 2009, 27, 6358–6362. [Google Scholar] [CrossRef]
- van Assen, S. Influenza Vaccination in Primary and Secondary Immunodeficiencies; Ipskamp Drukkers: Enschede, The Netherlands, 2011. [Google Scholar]
- Milanovic, M.; Stojanovich, L.; Djokovic, A.; Contic, M.; Gvozdenovic, E. Influenza vaccination in autoimmune rheumatic diseases patients. Tohoku J. Exp. Med. 2013, 229, 29–34. [Google Scholar] [CrossRef]
- de Lavallade, H.; Garland, P.; Sekine, T.; Hoschler, K.; Marin, D.; Stringaris, K.; Loucaides, E.; Howe, K.; Szydlo, R.; Kanfer, E.; et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica 2011, 96, 307–314. [Google Scholar] [CrossRef]
- Holvast, A.; Huckriede, A.; Wilschut, J.; Horst, G.; de Vries, J.J.C.; Benne, C.A.; Kallenberg, C.G.M.; Bijl, M. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann. Rheum. Dis. 2006, 65, 913–918. [Google Scholar] [CrossRef]
- van Assen, S.; Holvast, A.; Benne, C.A.; Posthumus, M.D.; van Leeuwen, M.A.; Voskuyl, A.E.; Blom, M.; Risselada, A.P.; de Haan, A.; Westra, J.; et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis. Rheum. 2010, 62, 75–81. [Google Scholar] [CrossRef]
- Kapetanovic, M.C.; Kristensen, L.E.; Saxne, T.; Aktas, T.; Mörner, A.; Geborek, P. Impact of anti-rheumatic treatment on immunogenicity of pandemic H1N1 influenza vaccine in patients with arthritis. Arthritis. Res. Ther. 2014, 16, R2. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, Y.J.; Shin, K.; Ha, Y.J.; Lee, E.Y.; Song, Y.W.; Choi, Y.; Winthrop, K.L.; Lee, E.B. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: A randomized clinical trial. Ann. Rheum. Dis. 2018, 77, 898–904. [Google Scholar] [CrossRef]
- Giacomelli, R.; Afeltra, A.; Alunno, A.; Bartoloni-Bocci, E.; Berardicurti, O.; Bombardieri, M.; Bortoluzzi, A.; Caporali, R.; Caso, F.; Cervera, R.; et al. Guidelines for biomarkers in autoimmune rheumatic diseases–evidence based analysis. Autoimmun. Rev. 2019, 18, 93–106. [Google Scholar] [CrossRef]
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, M.; Prymula, R. Influenza vaccination in the elderly. Hum. Vaccines Immunother. 2018, 14, 540–549. [Google Scholar] [CrossRef]
- Ng, T.W.Y.; Perera, R.A.P.M.; Fang, V.J.; Yau, E.M.; Peiris, J.S.M.; Tam, Y.H.; Cowling, B.J. The effect of influenza vaccination history on changes in hemagglutination inhibition titers after receipt of the 2015–2016 influenza vaccine in older adults in Hong Kong. J. Infect. Dis. 2020, 221, 33–41. [Google Scholar] [CrossRef]
- Darvishian, M.; van den Heuvel, E.R.; Bissielo, A.; Castilla, J.; Cohen, C.; Englund, H.; Gefenaite, G.; Huang, W.T.; la Bastide-van Gemert, S.; Martinez-Baz, I.; et al. Effectiveness of seasonal influenza vaccination in community-dwelling elderly people: An individual participant data meta-analysis of test-negative design case-control studies. Lancet Respir. Med. 2017, 5, 200–211. [Google Scholar] [CrossRef]
- Redondo, E.; Drago, G.; López-Belmonte, J.L.; Guillén, J.M.; Bricout, H.; Alvarez, F.P.; Callejo, D.; Gil de Miguel, Á. Cost-utility analysis of influenza vaccination in a population aged 65 years or older in Spain with a high-dose vaccine versus an adjuvanted vaccine. Vaccine 2021, 39, 5138–5145. [Google Scholar] [CrossRef]
- Giglio, N.D.; Castellano, V.E.; Mizrahi, P.; Micone, P.V. Cost-effectiveness of pneumococcal vaccines for adults aged 65 years and older in Argentina. Value Health Reg. Issues 2021, 28, 76–81. [Google Scholar] [CrossRef]
- Oláh, C.; Schwartz, N.; Denton, C.; Kardos, Z.; Putterman, C.; Szekanecz, Z. Cognitive dysfunction in autoimmune rheumatic diseases. Arthritis Res. Ther. 2020, 22, 78. [Google Scholar] [CrossRef]
- Liang, Y.; Meng, F.Y.; Pan, H.F.; Je, D.Q. A literature review on the patients with autoimmune diseases following vaccination against infections. Hum. Vaccines Immunother. 2015, 11, 2274–2280. [Google Scholar] [CrossRef][Green Version]
- Milanovic, M. Clinical and Serological Evaluation of the Vaccination against Seasonal Influenza in Autoimmune Rheumatic Diseases patients. Ph.D. Dissertation, Faculty of Medicine, Belgrade University, Belgrade, Serbia, 15 July 2013. [Google Scholar]
- Liao, Z.; Tang, H.; Xu, X.; Liang, Y.; Xiong, Y.; Ni, J. Immunogenicity and safety of influenza vaccination in systemic lupus erythematosus patients compared with healthy controls: A meta-analysis. PLoS ONE 2015, 11, e0147856. [Google Scholar] [CrossRef]
- Glück, T.; Müller-Ladner, U. Vaccination in patients with chronic rheumatic or autoimmune diseases. Clin. Infect. Dis. 2008, 46, 1459–1465. [Google Scholar] [CrossRef]
- Elkayam, O. Safety and efficacy of vaccination against influenza in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2006, 13, 349–351. [Google Scholar] [CrossRef]
- Nakafero, G.; Grainge, M.J.; Myles, P.R.; Mallen, C.D.; Zhang, W.; Doherty, M.; Nguyen-Van-Tam, J.S.; Abhishek, A. Association between inactivated influenza vaccine and primary care consultations for autoimmune rheumatic disease flares: A self-controlled case series study using data from the Clinical Practice Research Datalink. Ann. Rheum. Dis. 2019, 78, 1122–1126. [Google Scholar] [CrossRef]
- Nil, T.; Kubota, T.; Nanki, T.; Komano, Y.; Harigai, M.; Kohsaka, H.; Hirose, W.; Nagasaka, K.; Sakurai, T.; Miysaka, N. Reevaluation of antibody titers 1 year after influenza vaccination in patients with rheumatoid arthritis receiving TNF blockers. Mod. Rheumatol. 2009, 19, 216–218. [Google Scholar] [CrossRef]
- Stojanovich, L. Influenza vaccination of patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Clin. Dev. Immunol. 2006, 13, 373–375. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, Y.S.; Chen, W.S.; Chen, Y.H.; Chen, J.H. Effects of annual influenza vaccination on morbidity and mortality in patients with Systemic Lupus Erythematosus: A nationwide cohort study. Sci. Rep. 2016, 6, 37817. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, H.J.; Chen, W.S.; Lin, C.C.; Hsu, C.C.; Hsu, Y.H. Clinical effectiveness of influenza vaccination in patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2018, 21, 1246–1253. [Google Scholar] [CrossRef]
- Luque Ramos, F.; Hoffman, J.; Callhoff, A.; Zink, A.; Albrecht, K. Influenza and pneumococcal vaccination in patients with rheumatoid arthritis in comparison with age- and sex-matched controls: Results of a claims data analysis. Rheumatol. Int. 2016, 36, 1255–1263. [Google Scholar] [CrossRef]
- Harrison, N.; People, W.; Miksch, M.; Machold, K.; Kiener, H.; Aletaha, D.; Smolen, J.S.; Forstner, C.; Burgmann, H.; Lagler, H. Predictors for influenza vaccine acceptance among patients with inflammatory rheumatic diseases. Vaccine 2010, 36, 4875–4879. [Google Scholar] [CrossRef]
- Nguyen, M.; Lindegaard, H.; Hendricks, O.; Friis-Moller, N. Factors associated with influenza and pneumococcal vaccine uptake among rheumatoid arthritispatients in Denmark invited to participate in a pneumococcal vaccine trial (Immunovax_RA). Scand. J. Rheumatol. 2017, 46, 446–453. [Google Scholar] [CrossRef]
- Kadijevich, D.M.; Masliković, D.; Tomić, B.M. Familiarity with state regulations regarding access to information for persons with disabilities in Serbia. Int. J. Disabil. Dev. Educ. [Online early access]. Published Online: 23 August 2020. Available online: https://www.tandfonline.com/doi/abs/10.1080/1034912X.2020.1802646?journalCode=cijd20 (accessed on 27 December 2021). [CrossRef]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef]
- Abdelahad, M.; Ta, E.; Kesselman, M.M.; Demory Beckler, M. A review of the efficacy of influenza vaccination in autoimmune disease patients. Cureus 2021, 13, e15016. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanovic, M.S.; Kadijevich, D.M.; Stojanovich, L.; Milovanovic, B.; Djokovic, A. A Lower Level of Post-Vaccinal Antibody Titer against Influenza Virus A H1N1 May Protect Patients with Autoimmune Rheumatic Diseases from Respiratory Viral Infections. Medicina 2022, 58, 76. https://doi.org/10.3390/medicina58010076
Milanovic MS, Kadijevich DM, Stojanovich L, Milovanovic B, Djokovic A. A Lower Level of Post-Vaccinal Antibody Titer against Influenza Virus A H1N1 May Protect Patients with Autoimmune Rheumatic Diseases from Respiratory Viral Infections. Medicina. 2022; 58(1):76. https://doi.org/10.3390/medicina58010076
Chicago/Turabian StyleMilanovic, Milomir S., Djordje M. Kadijevich, Ljudmila Stojanovich, Branislav Milovanovic, and Aleksandra Djokovic. 2022. "A Lower Level of Post-Vaccinal Antibody Titer against Influenza Virus A H1N1 May Protect Patients with Autoimmune Rheumatic Diseases from Respiratory Viral Infections" Medicina 58, no. 1: 76. https://doi.org/10.3390/medicina58010076
APA StyleMilanovic, M. S., Kadijevich, D. M., Stojanovich, L., Milovanovic, B., & Djokovic, A. (2022). A Lower Level of Post-Vaccinal Antibody Titer against Influenza Virus A H1N1 May Protect Patients with Autoimmune Rheumatic Diseases from Respiratory Viral Infections. Medicina, 58(1), 76. https://doi.org/10.3390/medicina58010076






