Cognitive Behavioral Therapy for Migraine Headache: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Eligibility Criteria
2.3. Outcome Assessment and Data Extraction
2.4. Assessment of Risk of Bias
2.5. Data Synthesis
2.6. Heterogeneity and Subgroup Analysis
3. Results
3.1. The Results of Literature Search and Screening
3.2. Description of the Included Studies
3.3. Risk of Bias in the Included Studies
3.4. Primary Outcome
3.4.1. Headache Frequency
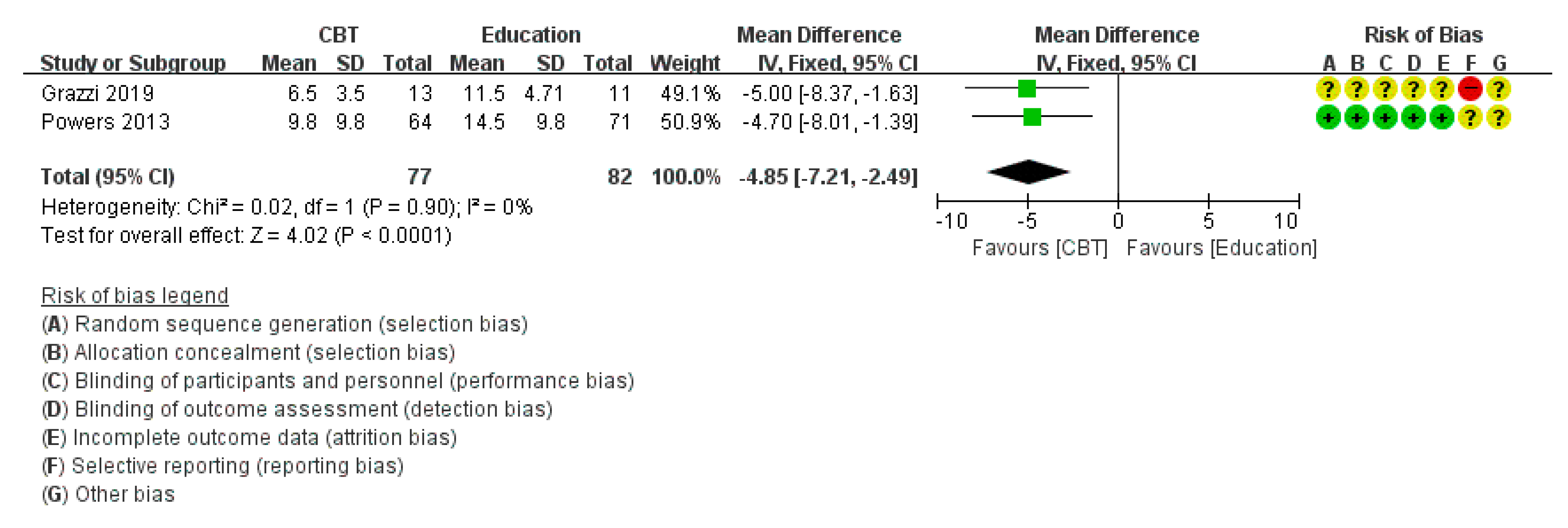
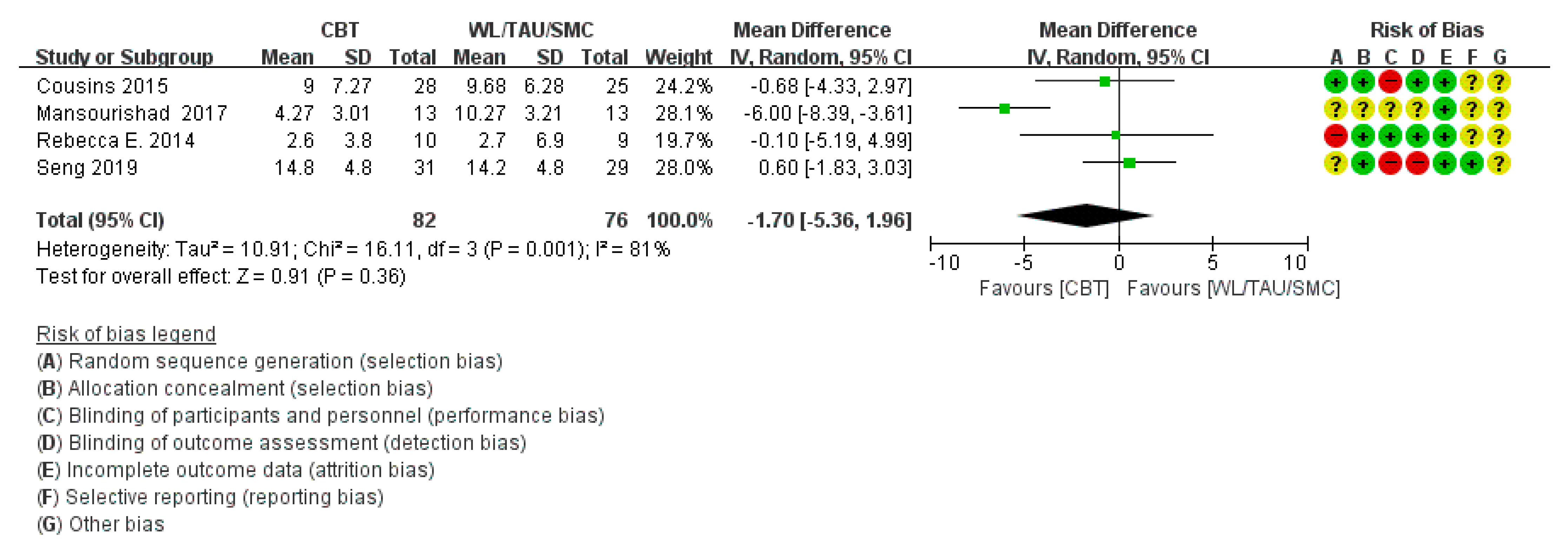
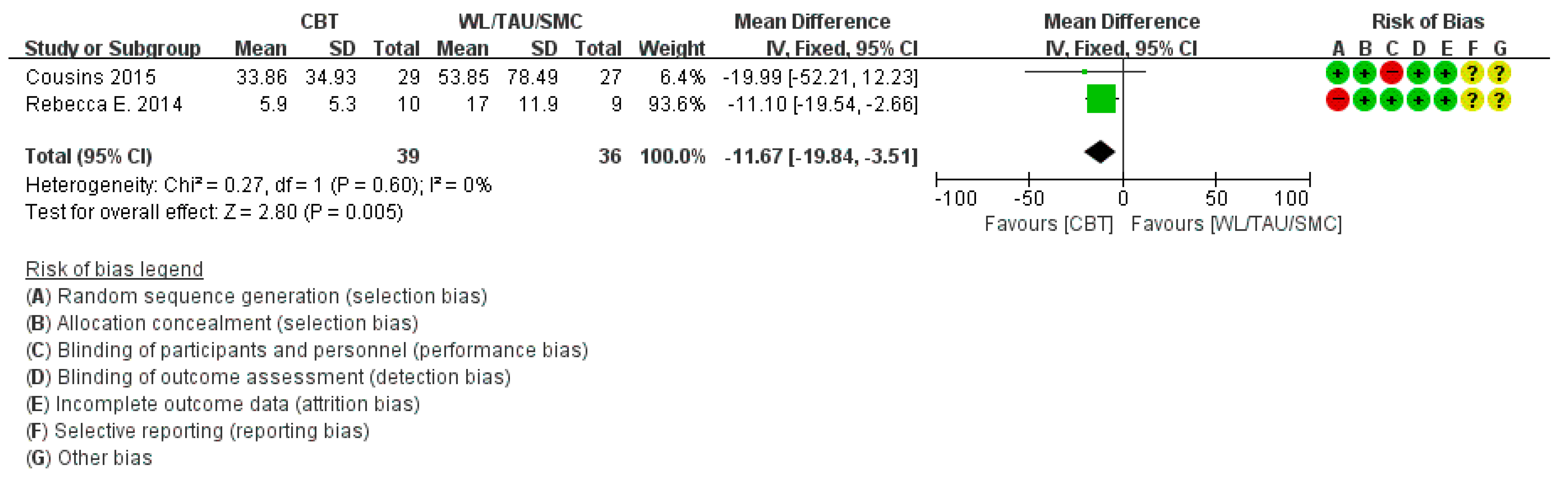
3.4.2. Migraine Disability Assessment Score
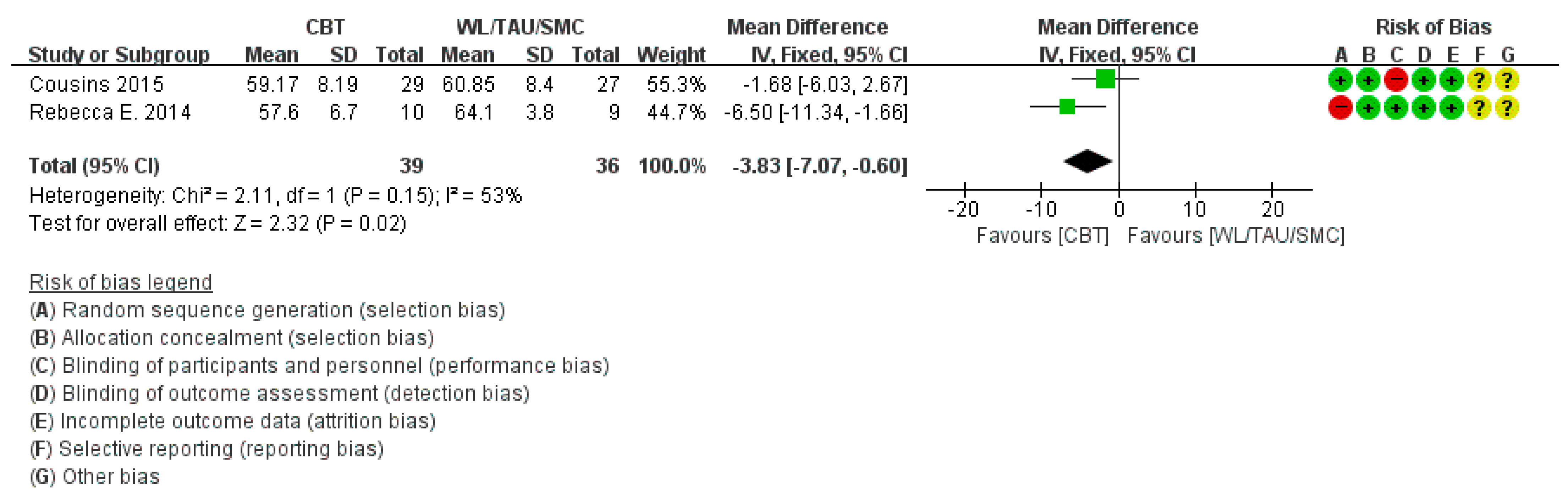
3.4.3. Headache Impact Test Score
3.5. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy According to DB
Appendix A.1. Medline
Appendix A.2. CENTRAL (The Cochrane Library)
- #1.
- MeSH descriptor: [Migraine Disorders] explode all trees
- #2.
- MeSH descriptor: [Headache] explode all trees
- #3.
- Headache or ‘Migraine Disorders’ or headache* or migrain* or cephalgi* or cephalalgi* or “migraine attack” or ‘episodic migraine’:ti,ab,kw (Word variations have been searched)
- #4.
- #1 or #2 or #3
- #5.
- MeSH descriptor: [Cognitive behavioral therapy] explode all trees
- #6.
- MeSH descriptor: [Cognitive behavioral therapies] explode all trees
- #7.
- (“cognitive behavioral therapy”) or (“cognitive behavioral therapies”):ti,ab,kw
- #8.
- #5 or #6 or #7
- #9.
- #4 and #8
Appendix A.3. EMBASE
Appendix A.4. CNKI
| #1 | pian tou tong(mean migraine) |
| #2 | fa zuo xing Pian tou tong(mean paroxysmal migraine) |
| #3 | Pian tou tong ji xing fa zuo(mean acute migraine attack) |
| #4 | tou jing mai(mean cephalic vein) |
| #5 | Migraine |
| #6 | OR/#1–#5 |
| #7 | ren zhi xing wei liao fa(mean cognitive behavioral therapy) |
| #8 | Cognitive behavioral therapy |
| #9 | Cognitive behavioral therapies |
| #10 | OR/#7–#9 |
| #11 | sui ji(mean random) |
| #12 | dui zhao(mean controlled trial) |
| #13 | OR/#11–#12 |
| #14 | #6 AND #10 AND #13 |
Appendix A.5. KISS, NDSL, OASIS, DBPIA
References
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders third ed. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Lipton, R.B.; Hutchinson, S.; Ailani, J.; Reed, M.L.; Fanning, K.M.; Adams, A.M.; Buse, D.C. Discontinuation of Acute Prescription Medication for Migraine: Results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache 2019, 59, 1762–1772. [Google Scholar] [CrossRef]
- Van Den Brink, M.; Antoinette; MacGregor, E.A. Gender and Migraine; Springer International: Cham, Switzerland, 2019; pp. 1–15. [Google Scholar]
- Silberstein, S.D. Preventive migraine treatment. Contin. Lifelong Learn. Neurol. 2015, 21, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Lipton, R.B.; Ferrari, M.D. Migraine—Current understanding and treatment. N. Engl. J. Med. 2002, 346, 257–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashina, M.; Buse, D.C.; Ashina, H.; Pozo-Rosich, P.; Peres, M.F.P.; Lee, M.J.; Terwindt, G.M.; Singh, R.H.; Tassorelli, C.; Do, T.P.; et al. Migraine: Integrated approaches to clinical management and emerging treatments. Lancet 2021, 397, 1505–1518. [Google Scholar] [CrossRef]
- Marmura, M.J.; Silberstein, S.D.; Schwedt, T.J. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015, 55, 3–20. [Google Scholar] [CrossRef]
- Worthington, I.; Pringsheim, T.; Gawel, M.J.; Gladstone, J.; Cooper, P.; Dilli, E.; Aube, M.; Leroux, E.; Becker, W.J. Canadian Headache Society Guideline: Acute drug therapy for migraine headache. Can. J. Neurol. Sci. 2013, 40 (Suppl. 3), S1–S80. [Google Scholar] [CrossRef] [Green Version]
- Evers, S.; Áfra, J.; Frese, A.; Goadsby, P.J.; Linde, M.; May, A.; Sándor, P.S. EFNS guideline on the drug treatment of migraine—Revised report of an EFNS task force. Eur. J. Neurol. 2009, 16, 968–981. [Google Scholar] [CrossRef]
- Khan, J.; Al Asoom, L.I.; Al Sunni, A.; Rafique, N.; Latif, R.; Al Saif, S.; Almandil, N.B.; Almohazey, D.; AbdulAzeez, S.; Borgio, J.F. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed. Pharmacother. 2021, 139, 111557. [Google Scholar] [CrossRef]
- Wells, R.E.; Burch, R.; Paulsen, R.H.; Wayne, P.M.; Houle, T.; Loder, E. Meditation for migraines: A pilot randomized controlled trial. Headache 2014, 54, 1484–1495. [Google Scholar] [CrossRef]
- Jacobs, G.D. Clinical applications of the relaxation response and mind–body interventions. J. Altern. Complement. Med. 2001, 7 (Suppl. 1), S93–S101. [Google Scholar] [CrossRef]
- Wells, R.E.; Smitherman, T.A.; Seng, E.K.; Houle, T.T.; Loder, E.W. Behavioral and mind/body interventions in headache: Unanswered questions and future research directions. Headache 2014, 54, 1107–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, R.E.; Loder, E. Mind/Body and behavioral treatments: The evidence and approach. Headache 2012, 52 (Suppl. 2), 70–75. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Lauche, R.; Frawley, J.; Sibbritt, D.; Adams, J. Utilization of complementary and alternative medicine and conventional medicine for headache or migraine during pregnancy: A cross-sectional survey of 1835 pregnant women. Complement. Ther. Med. 2018, 41, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, D.F.; Saluja, H.S. Prophylaxis of migraine headaches with riboflavin: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liampas, I.N.; Siokas, V.; Aloizou, A.-M.; Tsouris, Z.; Dastamani, M.; Aslanidou, P.; Brotis, A.; Dardiotis, E. Pyridoxine, folate and cobalamin for migraine: A systematic review. Acta Neurol. Scand. 2020, 142, 108–120. [Google Scholar] [CrossRef]
- Liampas, I.; Siokas, V.; Brotis, A.; Dardiotis, E. Vitamin D serum levels in patients with migraine: A meta-analysis. Rev. Neurol. 2020, 176, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G.; Asnaani, A.; Vonk, I.J.J.; Sawyer, A.T.; Fang, A. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cogn. Ther. Res. 2012, 36, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.E.; Chambless, D.L. Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: A meta-analysis of effectiveness studies. J. Consult. Clin. Psychol. 2009, 77, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Silberstein, S.D. Practice Parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000, 55, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, A.; Cousins, S.; Ridsdale, L. Psychological interventions for migraine: A systematic review. J. Neurol. 2016, 263, 2369–2377. [Google Scholar] [CrossRef] [Green Version]
- Seng, E.K.; Singer, A.B.; Metts, C.; Grinberg, A.S.; Patel, Z.S.; Ma, M.M.; Ma, L.R.; Day, M.; Minen, M.T.; Lipton, R.B.; et al. Does mindfulness-based cognitive therapy for migraine reduce migraine-related disability in people with episodic and chronic migraine? A Phase 2b Pilot Randomized Clinical Trial. Headache 2019, 59, 1448–1467. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Venkatanarayanan, N.; Kumar, L. A systematic review and meta-analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache 2017, 57, 349–362. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stewart, W.F.; Lipton, R.B.; Dowson, A.J.; Sawyer, J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001, 56 (Suppl. 1), S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, M.; Bayliss, M.S.; Bjorner, J.B.; Ware, J.E., Jr.; Garber, W.H.; Batenhorst, A.; Cady, R.; Dahlöf, C.G.; Dowson, A.; Tepper, S. A six-item short-form survey for measuring headache impact: The HIT-6. Qual. Life Res. 2003, 12, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.W.; Kashikar-Zuck, S.M.; Allen, J.R.; LeCates, S.L.; Slater, S.K.; Zafar, M.; Kabbouche, M.A.; O’Brien, H.L.; Shenk, C.E.; Rausch, J.R.; et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: A randomized clinical trial. JAMA 2013, 310, 2622–2630. [Google Scholar] [CrossRef]
- Grazzi, L.; Bernstein, C.; Raggi, A.; Sansone, E.; Grignani, E.; Searl, M.; Rizzoli, P. ACT for migraine: Effect of acceptance and commitment therapy (ACT) for high-frequency episodic migraine without aura: Preliminary data of a phase-II, multicentric, randomized, open-label study. Neurol. Sci. 2019, 40 (Suppl. 1), 191–192. [Google Scholar] [CrossRef]
- Mansourishad, H.; Togha, M.; Borjali, A.; Karimi, R. Effectiveness of mindfulness-based cognitive-behavioral therapy on relieving migraine headaches. Arch. Neurosci. 2017, 4, e58028. [Google Scholar] [CrossRef] [Green Version]
- Smitherman, T.A.; Walters, A.B.; Davis, R.E.; Ambrose, C.E.; Roland, M.; Houle, T.; Rains, J.C. Randomized controlled pilot trial of behavioral insomnia treatment for chronic migraine with comorbid insomnia. Headache 2016, 56, 276–291. [Google Scholar] [CrossRef]
- Cousins, S.; Ridsdale, L.; Goldstein, L.H.; Noble, A.J.; Moorey, S.; Seed, P. A pilot study of cognitive behavioural therapy and relaxation for migraine headache: A randomised controlled trial. J. Neurol. 2015, 262, 2764–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapoff, M.A.; Connelly, M.; Bickel, J.L.; Powers, S.W.; Hershey, A.D.; Allen, J.R.; Karlson, C.W.; Litzenburg, C.C.; Belmont, J.M. Headstrong intervention for pediatric migraine headache: A randomized clinical trial. J. Headache Pain 2014, 15, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritsche, G.; Frettlöh, J.; Hüppe, M.; Dlugaj, M.; Matatko, N.; Gaul, C.; Diener, H.-C. Prevention of medication overuse in patients with migraine. Pain 2010, 151, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, A.H.; Ford, S. Behavioral sleep modification may revert transformed migraine to episodic migraine. Headache 2007, 47, 1178–1183. [Google Scholar] [CrossRef]
- Scharff, L.; Marcus, D.A.; Masek, B.J. A controlled study of minimal-contact thermal biofeedback treatment in children with migraine. J. Pediatr. Psychol. 2002, 27, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Cochrane: London, UK, 2011. [Google Scholar]
- Ehde, D.M.; Dillworth, T.M.; Turner, J.A. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am. Psychol. 2014, 69, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.A.; Holtzman, S.; Mancl, L. Mediators, moderators, and predictors of therapeutic change in cognitive–behavioral therapy for chronic pain. Pain 2007, 127, 276–286. [Google Scholar] [CrossRef]


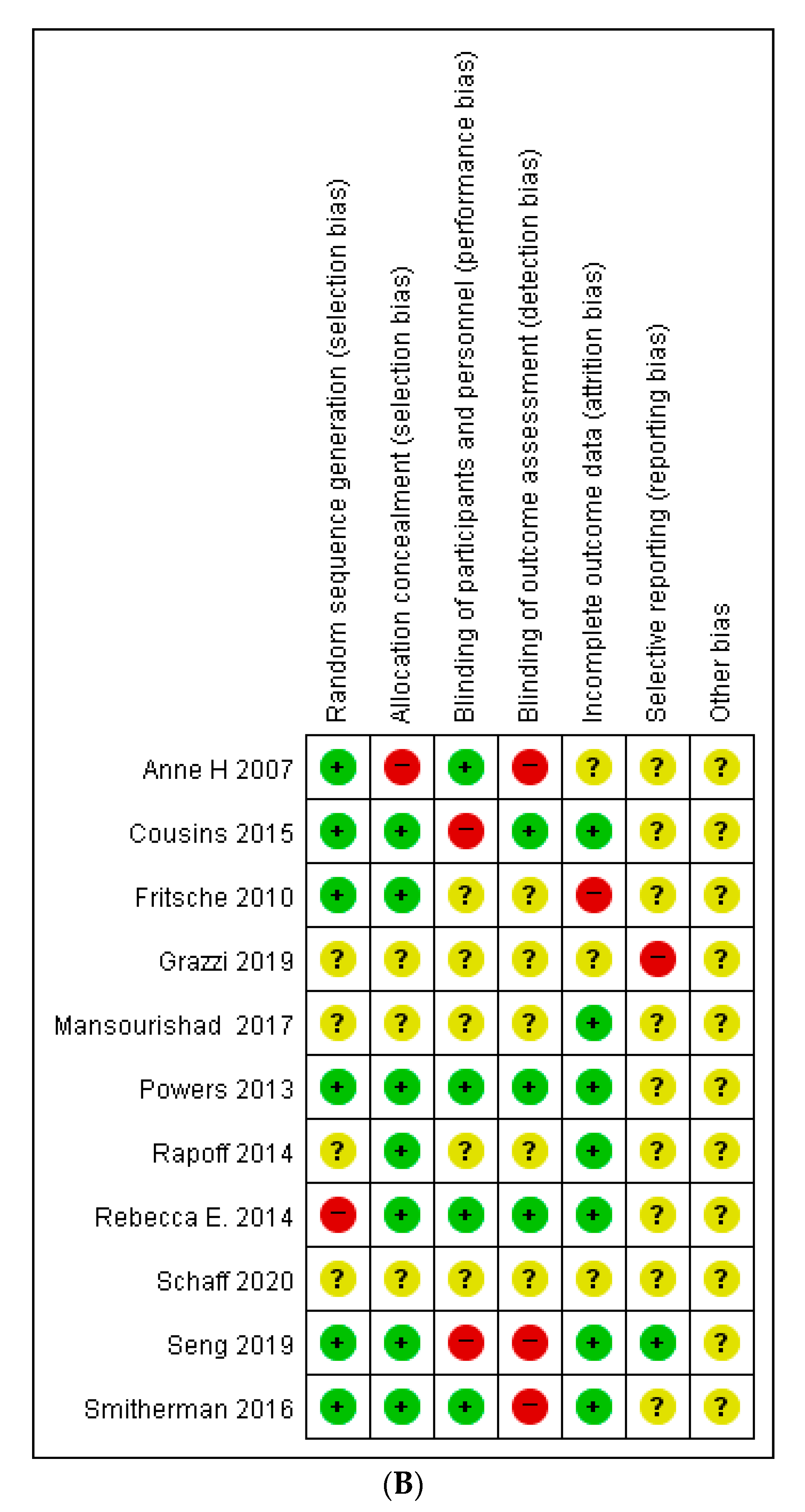

| Study ID (Year, Country) | Study Design | Age (Years, Mean ± SD) | Sex (n, Male/Female) | Inclusion Criteria | Exclusion Criteria | Intervention (n) | Control (n) | Duration | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| Powers [29] (2020, USA) | RCT | I: 14.4 ± 1.9 C: 14.4 ± 2.1 | I: 13/51 C: 15/56 | (1) ICHD-II - ≩15 days HA per month (HA diary) (2) PedMIDAS >20 - at least moderate disability | (1) Medication Overuse (ICHD-II) (2) Current use of amitriptyline or other prophylactic antimigraine medication within a period equivalent to less than 5 half-lives before study screening, (3) other chronic pain condition -fibromyalgia -complex regional pain syndrome II (4) Abnormal electrocardiogram (5) Severe orthostatic intolerance or dysregulation, (6) Documented developmental delay or impairment (7) Severe psychiatric comorbidity (eg, psychosis, bipolar disorder, major depressive disorder) (8) PedMIDAS > 140 (excessive disability and need for multisystemic therapies) (9) pregnancy or being sexually active without use of medically accepted form of contraception (barrier or hormonal methods) (10) use of disallowed medications including opioids, antipsychotics, antimanics, barbiturates, benzodiazepines, muscle relaxants, sedatives, tramadol, or herbal products. | CBT + Amitriptyline (64) | Education + Amitriptyline (71) | 20 weeks | 3, 6, 9 months |
| Seng [24] (2019, USA) | RCT | I: 36.2 ± 10.6 C: 44.2 ± 11.5 | I: 2/29 C: 3/26 | (1) ICHD-3 (2) Headache days ≥ 6 per month (3) Aged 18–65 years (4) Ability to read English (5) capacity to consent | (1) Continuous headache over the course of 30 days (2) Initiation of preventive migraine treatment within 4 weeks from baseline or during study (3) Severe psychiatric illness - active suicidality - active psychosis - falling cognitive screen (4) Inability to adhere to headache diary (recorded < 26/30 days) | MBCT-M (31) | WL/TAU (29) | 8–10 weeks (8 sessions) | 0, 1, 2, 4 months |
| Grazzi [30] (2019, Italy&USA) | RCT | I: 42.1 ± 11.6 C: 41.8 ± 11,1 | Not mentioned | (1) Age 18–65 years (2) High-frequency migraine w/o aura according to the IHS-beta-2013 criteria (3) No withdrawal intervention during 18 months | None | ACT + Education + Prophylaxis (13) | Education + Prophylaxis (11) | 6 weeks | 3, 6, 12 months |
| Mansourishad [31] (2017, Iran) | RCT | I: 33.6 ± 6.2 C: 30.7 ± 5.2 | I: 0/13 C: 0/13 | (1) ICHD-III criteria w or w/o aura (2) Age 20–40 years (3) Female (4) Minimum of 6 month gap between dignosis and beginnng of the study (5) Minimum of moderate IQ (6) No other psychological therapies over past 8 months (7) No use of medications for anxiety or depression during the past 3 months | (1) Severe physical illness (2) Serious neurological disorders or symptoms of psychosis (3) Unwillingness to continue treatment (4) Risk of suicidal thoughts and attempts requiring urgent intervention | MBCT (13) | Control (13) | 6 weeks | |
| Smitherman [32] (2016, USA) | RCT | I: 29.6 ± 13.4 C: 32.1 ± 12.8 | I: 1/15 C: 2/13 | Chronic Migraine comorbid insomnia (1) ICHD-II w/o MOH (2) ICSD-3 for insomnia | (1) Secondary headache dosorder including MOH (2) Pregnancy or breastfeeding (3) Being unable to read or speak English at a 6th grade level (4) Untreated sleep apnea (5) Active alcohol or substance use or dependence (6) Active bipolar disorder (7) Psychiatric hospitalization within last year (8) Employment involving rotating shift work schedule (9) Recent or expected change in preventive headache pharmacotherapy | CBTi (16) | Sham control (Lifestyle modification) (15) | Baseline 2 weeks + 6 weeks (biweekly) | 2, 6 weeks after Tx |
| Cousins [33] (2015, UK) | RCT | I: 40.67 ± 12.79 C: 37.97 ± 12.04 | I: 8/28 C: 5/32 | (1) 18–75 years (2) Onset >6 months ago (3) Day >3 HA days per month - assessed by HA diary - including episodic, chronic HA (4) IHS 2nd criteria - w or w/o aura | (1) Secondary HA (physical conditions lilely to cause HA) (2) Pregnancy (3) Current psychotic illness (4) Substance dependency (not including headache rescue medication) (5) Currently undergoing psychological therapy (6) inability to complete self-report measures | CBT + Relaxation + SMC (37) | SMC (36) | 5 weeks | 2, 4 months |
| Rapoff [34] (2014, USA) | RCT | I: 10.2 ± 2.0 C: 10.2 ± 1.5 | I: 8/10 C: 2/15 | (1) ICHD-II (w or w/o aura) (2) 7–12 years (3) Migraine occurring on the average at least once per week (by parental or child report and separated by symptom-free periods) | (1) Secondary headaches (2) Mental health condition or was receiving concurrent psychotherapy (3) CBC were in the clinical range at baseline (4) Average HA frequency <1 per week(over a 14-day period) | CD-Rom Headstrong (18) | CD-Rom Education (17) | 4 weeks | 3 months |
| Wells [12] (2014, USA) | RCT | I: 45.9 ± 17 C: 45.2 ± 12 | I: 1/9 C: 1/8 | (1) Migraine w or w/o aura (ICHD-II criteria) (2) 4–14 migraine days/month (3) ≥1 yr history of migraines (4) ≥ 18 years old (5) No additional disease | (1) Current regular meditation/yoga (2) Major systemic illness or unstable medical psychiatric condition (eg, suicide risk) (3) MOH (ICHD-II) (4) Current/planned pregnancy or breastfeeding (5) New prophylactic migraine medicine started within 4 weeks of the screening visit (6) Unwilling to maintain stable migraine medication dosages (7) Failure to complete baseline headache logs | MBSR (10) | TAU (9) | 8 weeks | |
| Fritche [35] (2010,Germany) | RCT | I: 47.7 ± 8.9 C: 48.4 ± 10.1 | I: 5/74 C: 9/62 | (1) ICHD-II w/o MOH (2) Migraine w and w/o aura (3) Combined HA (migraine + tension-type HA) - if migraine was main headache (4) 18–65 years (5) Intake - triptans on >4 and <10 days per month or - analgesics on >7 and <14 days per month during the past 3 months -combined triptans + analgesics ≨15 intake days including a maximum of 9 triptan intake days | (1) Significant psychiatric disorder (2) Additional secondary headache (3) Additional chronic pain diseases with pharmacological treatment (4) Insufficient knowledge of the German language. (5) Pregnancy | MCT + TAU (79) | Bibliotherapy (information brochures) + TAU (71) | 5 weeks | 3, 12–30 months |
| Calhoun [36] (2007, USA) | RCT | I: 33.5 C: 35.0 | I: 0/23 C: 0/20 | (1) IHS criteria (2) Adult women | (1) Non-pregnant (2) Non-lactating (3) Diagnosis of a primary sleep disorder | BSM (23) | Sham control (Placebo behavioral group) (20) | 2 + 6 weeks | 6, 12 weeks |
| Scarff [37] (2002, USA) | RCT | I (HWB): 13.3 ± 2.5 C-1 (HCB): 13.2 ± 2.0 C-2 (WLC): 12.0 ± 2.7 | I (HWB): 9/4 C-1 (HCB): 5/6 C-2 (WLC): 10/2 | (1) 7–17 years (2) IHS criteria w or w/o aura (3) No Primary medical condition and a negative neurological exam (4) Nor taking daily preventative medication for headaches (5) Average migraine ≥1 per week or ≥ 5 days per month | None | HWB (13) | (1) HCB (11) (2) WLC (12) | 6 weeks | 3, 6 months |
| Study ID | Treatment | Methods |
|---|---|---|
| Powers [29] (2020, USA) | I: CBT | Based on coping skills for pediatric pain, modified to include a biofeedback component. (thermal and electromyographic monitoring of the relaxation response) |
| C: Education | Consisted of discussion of headache-related education topics | |
| Seng [24] (2019, USA) | MBCT | MBCT protocols by Day et al. [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,31] (1) Automatic pilot: Practice (Body scan, Mindful eating), Homework (Body scan) (2) Awareness of appraisals and stress: Practice (Body scan, Awareness of appraisals, Awareness of thoughts arising during breathing meditation), Homework (Body scan, Mindfulness breath, Awareness of thoughts, Stress events calendar) (3) Mindfulness of the breath: Practice (Breathing space, Labeling automatic thoughts), Homework (Sitting meditation and body scan, Breathing space) (4) Recognizing aversion: Practice (Mindful movement; walking and stretching), Homework (Sitting meditation and mindful movement, Breathing space) (5) Allowing/Letting be: Practice (Identifying automatic thoughts, Sitting meditation with acceptance), Homework (Sitting meditation, Breathing space) (6) Thoughts are not facts: Practice (Sitting meditation, Mindful observation of cognitions and considering alternatives, Awareness of pleasant events), Homework (Choose your own meditation, Breathing space for coping, Pleasant events calendar (7) How can I best take care of myself?: Practice (Sitting meditation, Linking activity and mood/stress/migraine, Identifying warning signs for stress and migraine, Making a plan for nourishing activities), Homework (Develop routine to practice mindfulness, Dealing with stress and migraine) (8) Using Mindfulness to cope with migraine: Practice (Body scan, Relapse prevention, Focused meditation) |
| Grazzi [30] (2019, Italy&USA) | ACT | ○ Objective: patients were trained to practice mindfulness and pain management - psychological flexibility by cultivating six different positive capacities: acceptance, diffusion, sense of self, mindfulness, values, committed actions. - These capacities can improve mental and physical states, disability, and impact in pain conditions (resilience, avoidance, behavior, acceptance). ○ Sessions involved psycho education, discussions, experiential exercises, and home assignments. (1) Session 1: Creative helplessness; the problem of control (2) Session 2: Identifying values; introduction to mindfulness (3) Session 3: Actions guided by values; working with thoughts (4) Session 4: Working with acceptance/willingness (5) Session 5: Committed action; self-as-contest (6) Session 6: Integration; working with obstacles, wrap-up. (7) Final sessions 7/8: Integration; working with what was learned, exercises, wrap-up, practice at home. |
| Education | Education of patients, followed by pharmacological prophylaxis for migraine - topiramate, or propanolol, or amytriptiline according to the physician’s choice based on the patient profile, such as previous failures and contraindications. | |
| Mansourishad [31] (2017, Iran) | MBSR | - The sessions focused on developing nonjudgmental thinking and present-moment awareness of thoughts, emotions, and environment. - Homework assignments with the aid of a guided audio file included daily mindfulness meditation practices, such as body scan and breath awareness. - Session-by-session description of the protocol is available in the MBCT manual |
| Smitherman [32] (2016, USA) | CBTi | (1) Session 1: Included a detailed overview and rationale of the treatment components with instructions for daily home practice. (2) Sessions 2, 3 - Entailed reviewing daily diaries and treatment adherence since the last session, reinforcing progress, and problem-solving around any obstacles to adherence. - Participants continued daily self-monitoring throughout treatment and were instructed to continue practicing their 5 treatment instructions after treatment concluded - For insomnia (1) Go to bed only when sleepy and intending to sleep. (2) Leave the bedroom if unable to sleep after 20 min and return only when sleepy. (3) Use the bedroom only for sleep and sexual activity. (4) Set an alarm and rise daily at the same time. (5) Restrict your time in the bed to your total sleep time plus 30 min. - Sleep hygiene: promoting healthy sleep behaviors and sleep-conducive environmental conditions (e.g., limiting caffeine and meals prior to bedtime, keeping a comfortable bedroom temperature). |
| Sham control | Lifestyle modification (1) Eat dinner at a consistent time every evening. (2) Do acupressure (as instructed) for at least 2 min twice daily, once on awakening and once before going to bed. (3) Record all liquids consumed for 3 consecutive “typical” days (identity of liquid, quantity, and time of day) and thereafter keep a consistent liquid intake each day. (4) Do 5 min of stretching/range of motion exercises upon awakening. (5) Consume at least one serving of protein within one hour of arising in the morning (e.g., egg, cheese, cottage cheese, and tofu). | |
| Cousins [33] (2015, UK) | I: CBT +Relaxation C: SMC | (1) Week 1: [Session 1] Introducing the concept of links between thoughts, feelings, symptoms, and behaviors, thought monitoring and relaxation techniques. - Expectations of treatment, Brief assessment, What is CBT, Introduction of headache/thought diary and relaxation techniques (2) Week 2: Telephone follow up: Reviews progress, offer support (3) Week 3: [Session 2] Problem solving and cognitive restructuring including alternative thinking - Review of headache/thought diaries and progress with relaxation techniques - Introduction of problem solving and cognitive restructuring (4) Week 4: Telephone follow up: Reviews progress, offer support (5) Week 5: [Session 3] building on alternative thinking techniques and covered relapse prevention - Review the progress with relaxation/cognitive restructuring techniques (using headache/thought diary) - Problem solving - Ways to maintain improvement |
| Wells [12] (2014, USA) | MBSR | MBSR protocol by Dr. Jon Kabat-Zinn [7,8,9,10,11,12,13,14,15] - begins with mindfulness of breathing, mindful eating, and the body scan. - subsequent classes build on these practices and slowly add in the other meditative practices. - The all-day retreat includes elements of all the mindfulness practices. - The instructor also gives information about stress and stress relief during the fourth class. |
| Rapoff [34] (2014, USA) | I: Headstrong | (1) Week 1: Headache education & cognitive behavioral model of pain - Introduction, Types of headache, Prevalence of headache, Features of headache, How headache is diagnosed, The pain puzzle, Headache triggers (2) Week 2: Relaxation - Rationale for relaxation, How to use guided imagery, How to use deep breathing, How to use progressive muscle relaxation (3) Week 3: Cognitive restructuring - Rationale for coping, Thought-out changing, Problem-solving (4) Week 4: Pain behaviors - Positive and negative pain behaviors, Importance of keeping active, Review of all lesson |
| C: Education | (1) Week 1: Headache education (Introduction, Types of headache, Prevalence of headache, Features of headache, How headache is diagnosed) (2) Week 2: Cognitive-behavioral model of pain (Introduction to the pain puzzle, puzzle piece-1; nociception, 2; thoughts) (3) Week 3: Cognitive-behavioral model of pain (puzzle piece-3; feelings, 4; behavior) (4) Week 4: Headache triggers (Introduction to headache triggers, key headache triggers, diet, and sleep) | |
| Fritche [35] (2010, Germany) | MCT | (1) Unit 1: Introduction and syndrome education - information about symptoms, pathophysiology and pathopsychology of migraine as well as instructions for progressive muscle relaxation (PMR) (2) Unit 2: Medication rules and the risk of Medication Overuse Headache - information about acute and prophylactic migraine medication and MOH-symptoms and pathomechanisms - establish a clear behavioral intake algorithm in migraine attack situations, (3) Unit 3: Medication intake behavior - aimed at raising awareness for ‘external’ (e.g., availability of drugs, stock-keeping, iatrogenic risk factors like doctor shopping) and ‘internal’ (e.g., fear of attack and losing social functioning, stress level in private and professional life) influences on patient’s medication intake behavior. (4) Unit 4: General and personal risk factors for drug intake - established a general risk profile of medication overuse for each patient. (5) Unit 5: Everyday transfer - aim to establish individual goals for future drug intake and learning how to make use of social support to control intake behavior. (6) Daily PMR and Headache Diary: Daily exercise of PMR as well as keeping a daily headache diary had to be performed during the time between all five sessions (7) Homework: after each session, patients were given topic-related homework. |
| Calhoun [36] (2007, USA) | BSM Instructions | (1) Schedule consistent bedtime that allows 8 h in bed (2) Eliminate TV, reading, music in bed (3) Use visualization technique to shorten time to sleep onset (4) Move supper ≥4 h before bedtime; limit fluids within 2 h of bedtime (5) Discontinue naps |
| Sham Instructions | (1) Schedule consistent suppertime that varies <1 h from day to day (2) Perform acupressure as instructed for 2 min twice daily (3) Record liquid consumption for 3 consecutive days (4) Do 5 min of gentle range of motion exercises every morning (5) Have 1 protein serving at breakfast | |
| Scarff [37] (2002, USA) | I: CBT + HWB C-1: CBT + HCB C-2: WLC | (1) Cognitive behavioral stress management training - younger children (under 13 years) were taught thought stopping and positive self-statements. - Older children were trained to identify stressful thoughts that triggered migraine episodes, test the logic of their thoughts in a more formal manner. (2) Thermal biofeedback training(hand-warming) (3) Progressive muscle relaxation (4) Imagery training of warm places and vasodilation (5) instruction in deep breathing techniques |
| Study ID | Main Result | Baseline (BL) | Post-Treatment (PT) | Follow-Up (F/U) | p-Value | Adverse Effect | Conclusion | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (Mean ± SD) | ||||||||||||
| Control (Mean ± SD) | ||||||||||||
| Powers [29] (2013) | Group MD in change score at PT | I:90 C:109 | ○ In I group, headache days and PedMIDAS decreased significantly compared with control group. ○ Headache days were ≥50% Reduction in 66% of I and 36% of C at PT (odds ratio, 3.5 [95%CI, 1.7 to 7.2]; p < 0.001). ○ PedMIDAS <20 points were in 75% of I, and 56% of C at PT (odds ratio, 2.4 [95%CI, 1.1 to 5.1]; p = 0.02). | |||||||||
| HA frequency (day/month) | I | 21.3 ± 5.2 | 9.8 ± 9.8 | Not mentioned | p = 0.002 | |||||||
| C | 21.3 ± 5.2 | 14.5 ± 9.8 | ||||||||||
| PedMIDAS | I | 68.2 ± 31.7 | 15.5 ± 17.4 | Not mentioned | 14.1 (95% CI 3.3, 24.9) p = 0.01 | |||||||
| C | 68.2 ± 31.7 | 29.6 ± 42.2 | ||||||||||
| Seng [24] (2019) | 4 M | ○ No AE in control group ○ 2 AE in intervention group (1) Vivid recollection of traumatic event while practicing mindfulness (2) Severe increase in Headache frequency and pain intensity | ○ HDI change: the group*month interaction was significant, p = 0.004 ○ MIDAS: Group*month (N.S. accounting for divided alpha) p = 0.027 ○ MIDI: −0.6/10(I), +0.3/10(C) p = 0.007 ○ Headache days, headache intensity: Group*month interaction and group*time interaction (N.S.) | |||||||||
| HDI 1 | I | 52.5 ± 21.2 | 38.2 ± 16.6 | p < 0.004 | ||||||||
| C | 50.2 ± 16.2 | 50.4 ± 14.3 | ||||||||||
| MIDAS 2 | Group*month interaction BL vs. 4 M | B = 1.6, 95%CI = −0.7,3.9 F(3,213) = 3.12, p = 0.027 | ||||||||||
| MIDAS-A 3 | B = 6.3, 95%CI = −2.0,14.5, F(3,94.6) = 1.21, p = 0.312 | |||||||||||
| MIDAS-B 4 | B = 0.3, 95%CI = −0.4,1.1, F(3,102.9) = 0.65, p = 0.589 | |||||||||||
| HA days/month | I | 16.5 ± 6.0 | 14.8 ± 4.8 | p = 0.773 | ||||||||
| C | 15.5 ± 5.9 | 14.2 ± 4.8 | ||||||||||
| Average Attack Intensity /month 5 | I | 1.7 ± 0.3 | 1.6 ± 0.3 | p = 0.888 | ||||||||
| C | 1.8 ± 0.3 | 1.7 ± 0.3 | ||||||||||
| Average MIDI/month 6 | I | 2.8 ± 1.6 | 1.7 ± 2.7 | p = 0.007 | ||||||||
| C | 3.4 ± 2.0 | 4.4 ± 1.3 | ||||||||||
| Grazzi [30] (2019) | 3 M | N | ○ Headache days and medication intake days declined in I group, not in C group | |||||||||
| HA days/month | I | 10 ± 2.0 | 6.5 ± 3.5 | N | ||||||||
| C | 9.27 ± 3.43 | 11.5 ± 4.71 | ||||||||||
| Using medication days/month | I | 9.4 ± 2.75 | 5.75 ± 3.3 | N | ||||||||
| C | 9.9 ± 3.6 | 10.5 ± 5.8 | ||||||||||
| Mansourishad [31] (2017) | 3 M | N | ○ Covariance analysis showed I group is effective compared with C group in reducing headache frequency (p= 0.001< 0.05), duration (p = 0.001 < 0.05), and severity (p= 0.001 < 0.05) in women with migraine. | |||||||||
| HA Frequency (days/month) | I | 10.63 ± 6.16 | 4.27 ± 3.01 | 4.73 ± 2.01 | p = 0.001 | |||||||
| C | 10.81 ± 4.56 | 10.27 ± 3.21 | 10.45 ± 6.07 | |||||||||
| HA Intensity | I | 6.20 ± 2.30 | 4.12 ± 1.90 | 4.32 ± 1.13 | p = 0.001 | |||||||
| C | 6.41 ± 3.40 | 6.40 ± 2.83 | 6.50 ± 2.75 | |||||||||
| HA Duration (h/month) | I | 10.63 ± 3.05 | 5.90 ± 4.29 | 5.08 ± 2.76 | p = 0.001 | |||||||
| C | 11.73 ± 5.49 | 12.45 ± 6.22 | 11.36 ± 4.85 | |||||||||
| PT | F/U | |||||||||||
| Smitherman [32] (2016) | HA Frequency (days/month) | I | 22.7 | 16.6 | 11.6 | p = 0.883 | p = 0.028 | N | ○ Headache frequency reduction from baseline of I group was not statistically significant com-pared with C group at PT, FU p = 0.883 ○ I and C group showed clinically meaningful re-ductions in MIDAS, HIT-6, headache severity at PT, FU with no group differences when controlling for baseline scores ○ No significant group difference in Headache frequency, HIT-6, MIDAS score, headache severity, ESS, PHQ-9, GAD-7, CEQ ○ Significant group difference in TST, sleep efficiency, PSQI | |||
| C | 19.6 | 12.5 | 14.7 | |||||||||
| MIDAS | I | 59.9 ± 39.0 | 44.2 ± 43.1 | 31.9 ± 33.2 | N | |||||||
| C | 54.5 ± 41.0 | 41.0 ± 46.2 | 34.7 ± 34.5 | |||||||||
| HIT-6 | I | 66.9 ± 3.8 | 62.6 ± 5.3 | 59.9 ± 5.5 | N | |||||||
| C | 64.8 ± 3.9 | 61.4 ± 8.0 | 59.6 ± 7.2 | |||||||||
| HA Severity | I | 5.2 ± 0.9 | 5.1 ± 1.4 | 4.5 ± 1.5 | N | |||||||
| C | 5.4 ± 1.6 | 5.2 ± 2.1 | 5.1 ± 1.9 | |||||||||
| PSQI 7 | I | 11.3 ± 4.4 | 7.6 ± 2.6 | 7.0 ± 3.1 | p = 0.009 | |||||||
| C | 11.6 ± 2.5 | 10.9 ± 3.8 | 11.5 ± 3.9 | |||||||||
| ESS 8 | I | 11.0 ± 3.4 | 9.0 ± 3.2 | 8.9 ± 3.55 | N | |||||||
| C | 9.9 ± 4.892 | 9.2 ± 4.7 | 8.8 ± 4.6 | |||||||||
| TST (h) 9 | I | 7.4 ± 1.5 | 7.3 ± 1.4 | 8.3 ± 2.6 | p = 0.049 | |||||||
| C | 6.7 ± 1.5 | 6.9 ± 1.2 | 6.8 ± 0.5 | |||||||||
| Sleep Efficiency | I | 81.2 ± 7.7 | 79.1 ± 8.9 | 84.9 ± 4.5 | p = 0.001 | |||||||
| C | 81.2 ± 8.3 | 82.4 ± 6.4 | 80.9 ± 4.9 | |||||||||
| PHQ-9 10 | I | 12.1 ± 5.8 | 6.9 ± 4.8 | 6.3 ± 4.6 | p = 0.054 | |||||||
| C | 10.5 ± 4.5 | 8.4 ± 4.7 | 8.6 ± 4.7 | |||||||||
| GAD-7 11 | I | 10.6 ± 6.4 | 6.6 ± 5.2 | 6.3 ± 4.8 | p = 0.430 | |||||||
| C | 9.8 ± 5.3 | 7.0 ± 4.6 | 6.9 ± 4.9 | |||||||||
| Cousins [33] (2015) | 4 M | N | ○ At 4 months after treatment, no significant change between I and C group statistically in. - Diary headache days - Medication days/ month - MIDAS, HIT-6, HADS-A, - HADS-D, IPQ | |||||||||
| HA days/month | I | 12.03 ± 8.70 | 9 ± 7.27 | N | ||||||||
| C | 11.54 ± 6.64 | 9.68 ± 6.28 | ||||||||||
| Using rescue medication days/month | I | 6.69 ± 5.30 | 5.86 ± 5.12 | N | ||||||||
| C | 7.08 ± 5.87 | 6.2 ± 4.86 | ||||||||||
| MIDAS | I | 51.03 ± 43.68 | 33.86 ± 34.93 | N | ||||||||
| C | 65.78 ± 46.79 | 53.85 ± 78.49 | ||||||||||
| HIT-6 12 | I | 66.5 ± 5.88 | 59.17 ± 8.19 | N | ||||||||
| C | 65.97 ± 4.41 | 60.85 ± 8.4 | ||||||||||
| HADS-A 13 | I | 7.78 ± 4.01 | 5.76 ± 4.45 | N | ||||||||
| C | 9.32 ± 3.55 | 7.96 ± 4.37 | ||||||||||
| HADS-D | I | 5.83 ± 4.61 | 4.24 ± 4.6 | N | ||||||||
| C | 5.68 ± 3.09 | 4.52 ± 3.51 | ||||||||||
| Brief IPQ 14 | I | 52.81 ± 9.69 | 44.17 ± 15.89 | N | ||||||||
| C | 51.41 ± 9.77 | 45.26 ± 10.17 | ||||||||||
| Wells [12] (2014) | PT | FU | N | ○ The severity and du-ration of all head-aches decreased in the I group, but not statistically significant ○ Significant decrease in I group compared with C group on HIT-6 at PT (p = 0.043), FU(p = 0.022) and MIDAS at PT (p = 0.017) ○ Self-efficacy and mindfulness also in-creased at PT (p = 0.035) ○ MBSR is safe and feasible for adults with migraine. | ||||||||
| Migraine Frequency /month | I | 4.2 ± 2.9 | 2.6 ± 3.8 | p = 0.38 | p = 0.63 | |||||||
| C | 2.9 ± 5.2 | 2.7 ± 6.9 | ||||||||||
| HA Frequency days/month | I | 9.9 * | 9.0 * | 9.0 * | p = 0.14 | p = 0.22 | ||||||
| C | 12.3 * | 10.0 * | 7.7 * | |||||||||
| HA severity (0–10) | I | 4.4 * | 3.2 * | 3.3 * | p = 0.053 | p = 0.66 | ||||||
| C | 4.8 * | 5.2 * | 4.8 * | |||||||||
| HA duration | I | 5.1 * | 2.9 * | 3.6 * | p = 0.043 | p = 0.19 | ||||||
| C | 6.4 * | 6.1 * | 6.1 * | |||||||||
| HIT-6 | I | 63.0 ± 8.0 | 57.6 ± 6.7 | 58.3 ± 6.0 | p = 0.043 | p = 0.022 | ||||||
| C | 64.7 ± 5.0 | 64.1 ± 3.8 | 64.1 ± 3.9 | |||||||||
| MIDAS | I | 12.5 ± 9.8 | 5.9 ± 5.3 | 5.8 ± 3.8 | p = 0.017 | p = 0.072 | ||||||
| C | 11.0 ± 6.7 | 17.0 ± 11.9 | 12.0 ± 8.1 | |||||||||
| HA Management Self Efficacy | I | 117.2 ± 18.7 | 122.6 ± 25.0 | 124.5 ± 22.6 | p = 0.035 | p = 0.060 | ||||||
| C | 118.4 ± 31.1 | 110.7 ± 29.2 | 111.9 ± 35.7 | |||||||||
| Five Factor Mindfulness | I | 142.9 ± 14.7 | 149.1 ± 18.7 | 153.8 ± 19.7 | p = 0.035 | p = 0.045 | ||||||
| C | 143.7 ± 20.3 | 136.8 ± 18.3 | 138.0 ± 19.6 | |||||||||
| MSQoL 15 | I | 47.0 * | 31.5 * | 38.1 * | p = 0.12 | p = 0.035 | ||||||
| C | 46.4 * | 45.2 * | 45.2 * | |||||||||
| PHQ-9 16 | I | 3.6 ± 3.0 | 2.0 ± 1.8 | 2.7 ± 2.2 | p = 0.77 | p = 0.59 | ||||||
| C | 6.4 ± 6.5 | 4.2 ± 1.8 | 3.9 ± 1.9 | |||||||||
| STAI 17 | I | 68.7 ± 16.3 | 61.6 ± 15.0 | 60.5 ± 16.8 | p = 0.13 | p = 0.10 | ||||||
| C | 67.0 ± 15.8 | 70.2 ± 14.9 | 69.1 ± 10.3 | |||||||||
| Perceived Stress Scale-10 | I | 15.8 ± 6.4 | 13.3 ± 5.1 | 12.1 ± 5.1 | p = 0.87 | p = 0.27 | ||||||
| C | 14 ± 8.1 | 12.1 ± 8.0 | 13.6 ± 7.0 | |||||||||
| Rapoff [34] (2014) | 3 M | BL | PT | N | ○ In I group, pain severity decreased at PT compared with C group (p = 0.03) ○ At 3 M FU, significant change in PedMIDAS score in I group compared with C group (p = 0.04) No other group differences at PT or 3M FU | |||||||
| HA frequency (% of days) | I | 41.09 ± 22.67 | 31.28 ± 28.14 | 21.43 ± 23.47 | p = 0.48 | p = 0.46 | p = 0.36 | |||||
| C | 40.67 ± 28.79 | 32.14 ± 22.23 | 18.18 ± 17.60 | |||||||||
| HA duration (hr/episode) | I | 5.47 ± 4.20 | 4.47 ± 4.26 | 1.53 ± 0.91 | p = 0.19 | p = 0.24 | p = 0.07 | |||||
| C | 4.15 ± 3.88 | 5.56 ± 4.01 | 4.25 ± 5.19 | |||||||||
| HA severity (VAS) | I | 5.06 ± 1.84 | 5.06 ± 1.50 | 4.46 ± 1.88 | p = 0.07 | p = 0.03 | p = 0.20 | |||||
| C | 6.00 ± 1.52 | 6.25 ± 1.92 | 3.68 ± 2.04 | |||||||||
| PedMIDAS total 18 | I | 13.26 ± 9.69 | 7.82 ± 10.59 | 0.91 ± 1.45 | p = 0.25 | p = 0.14 | p = 0.05 | |||||
| C | 15.53 ± 10.08 | 12.29 ± 12.94 | 3.50 ± 4.86 | |||||||||
| PedsQL total 19 | I | 82.10 ± 12.18 | 83.70 ± 12.07 | 84.88 ± 18.22 | p = 0.25 | p = 0.26 | p = 0.46 | |||||
| C | 79.35 ± 11.55 | 80.69 ± 14.36 | 85.67 ± 14.32 | |||||||||
| Fritsche [35] (2010) | 3 M | 12–30 M | N | ○ Significant change in time effect observed in I, C group in headache days, migraine days, Intake at headache days, Intake at migraine days (p < 0.001) ○ Improvement in psychological variables (p < 0.001) - Remained stable in both groups at short- and long-term F/U ○ MCT (C) and biblio-therapy (I) are useful to prevent “medication overuse headache” and transition to chronic head-ache | ||||||||
| HA days | I | 11.40 ± 5.92 | 9.17 ± 5.45 | 8.55 ± 5.51 | 8.68 ± 5.29 | Time effect p < 0.001 | ||||||
| C | 10.51 ± 4.98 | 8.47 ± 5.54 | 8.11 ± 4.82 | 8.33 ± 5.15 | ||||||||
| Migraine days | I | 7.23 ± 3.70 | 5.60 ± 3.79 | 6.15 ± 3.97 | 6.15 ± 4.02 | Time effect p < 0.001 | ||||||
| C | 7.27 ± 3.82 | 5.78 ± 4.01 | 5.45 ± 3.16 | 5.84 ± 3.76 | ||||||||
| HA disability | I | 4.46 ± 1.80 | 4.49 ± 2.01 | 4.61 ± 1.97 | 4.39 ± 2.16 | Time effect N.S | ||||||
| C | 4.16 ± 1.56 | 4.13 ± 1.97 | 4.25 ± 1.88 | 4.40 ± 1.73 | ||||||||
| Intake at HA days | I | 7.17 ± 2.48 | 5.92 ± 3.10 | 5.93 ± 3.23 | 6.18 ± 3.65 | Time effect p < 0.001 | ||||||
| C | 7.58 ± 3.11 | 6.35 ± 3.66 | 6.47 ± 3.20 | 6.00 ± 2.82 | ||||||||
| Intake at migraine days | I | 5.27 ± 2.25 | 4.30 ± 2.76 | 4.83 ± 3.00 | 5.03 ± 3.52 | Time effect p < 0.001 | ||||||
| C | 6.25 ± 2.98 | 5.04 ± 3.11 | 4.75 ± 2.82 | 5.02 ± 2.78 | ||||||||
| Calhoun [36] (2007) | HA frequency /28 days | I | 24.2 ** | 17.4 ** | p = 0.001 | N | ○ In I group, statistically significant reduction compared to C group observed headache frequency (p = 0.001) and Headache intensity(p= 0.01) at PT ○ No one in C group re-verted to episodic migraine, and 48.5% in I group reverted to epi-sodic migraine | |||||
| C | 23.2 ** | 23.9 ** | ||||||||||
| HA Intensity | I | 46.7 ** | 28.3 ** | p = 0.01 | ||||||||
| C | 50.2 ** | 44.1 ** | ||||||||||
| Reverted to episodic migraine | p = 0.029 | |||||||||||
| Scharff [37] (2002) | PT | F/U | N | ○ Clinical improvement in all variables (head-ache index change, highest intensity, days with headache) over time and compared with C group. ○ In HWB and HCB group ○ At PT, there is no significant temperature change ○ At 3 M F/U, temperature changes in both HWB and HCB group were significant com-pared to BL ○ At 6 M F/U, HWB group showed clinical improvement compared to HCB group | ||||||||
| HA Index change | (1) Effect of time (Pillai’s trace = 0.267, F[3, 29] = 3.53, p < 0.03) (2) Trend for treatment group (Pillai’s trace = 0.36, F[6, 60] = 2.21, p < 0.05) | (1) Effect for time (Pillai’s trace =0.81, F[9, 12] = 5.62, p < 0.01) (2) Trend for treatment group (Pillai’s trace = 0.32, F[3, 18] = 2.80, p < 0.07) | p < 0.005 | p < 0.001 | ||||||||
| Highest intensity rating for 2-week | p < 0.01 | N | ||||||||||
| HA days | p < 0.02 | p < 0.01 | ||||||||||
| Temperature change | (1) Effect of time (Philai’s trace = 0.44, F[12, 69] = 4.44, p < 0.001) (2) No significant difference in treatment | 6 M | N | |||||||||
| 72.2% of HWB 20, 33.3% of HCB 21 were significant compared to BL (χ² [1] = 3.76, p < 0.05). | 100% of HWB 62.5% of HCB showed clinical improvement (χ² [1] = 4.50, p < 0.05). | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.-y.; Sung, H.-K.; Kwon, N.-Y.; Go, H.-Y.; Kim, T.-j.; Shin, S.-M.; Lee, S. Cognitive Behavioral Therapy for Migraine Headache: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 44. https://doi.org/10.3390/medicina58010044
Bae J-y, Sung H-K, Kwon N-Y, Go H-Y, Kim T-j, Shin S-M, Lee S. Cognitive Behavioral Therapy for Migraine Headache: A Systematic Review and Meta-Analysis. Medicina. 2022; 58(1):44. https://doi.org/10.3390/medicina58010044
Chicago/Turabian StyleBae, Ji-yong, Hyun-Kyung Sung, Na-Yoen Kwon, Ho-Yeon Go, Tae-jeong Kim, Seon-Mi Shin, and Sangkwan Lee. 2022. "Cognitive Behavioral Therapy for Migraine Headache: A Systematic Review and Meta-Analysis" Medicina 58, no. 1: 44. https://doi.org/10.3390/medicina58010044
APA StyleBae, J.-y., Sung, H.-K., Kwon, N.-Y., Go, H.-Y., Kim, T.-j., Shin, S.-M., & Lee, S. (2022). Cognitive Behavioral Therapy for Migraine Headache: A Systematic Review and Meta-Analysis. Medicina, 58(1), 44. https://doi.org/10.3390/medicina58010044






