Safety of Aesthetic Medicine Procedures in Patients with Autoimmune Thyroid Disease: A Literature Review
Abstract
1. Introduction
2. Hyaluronic Acid
3. Botulinum Toxin
4. Autologous Platelet-Rich Plasma
5. Autologous Fat Grafting
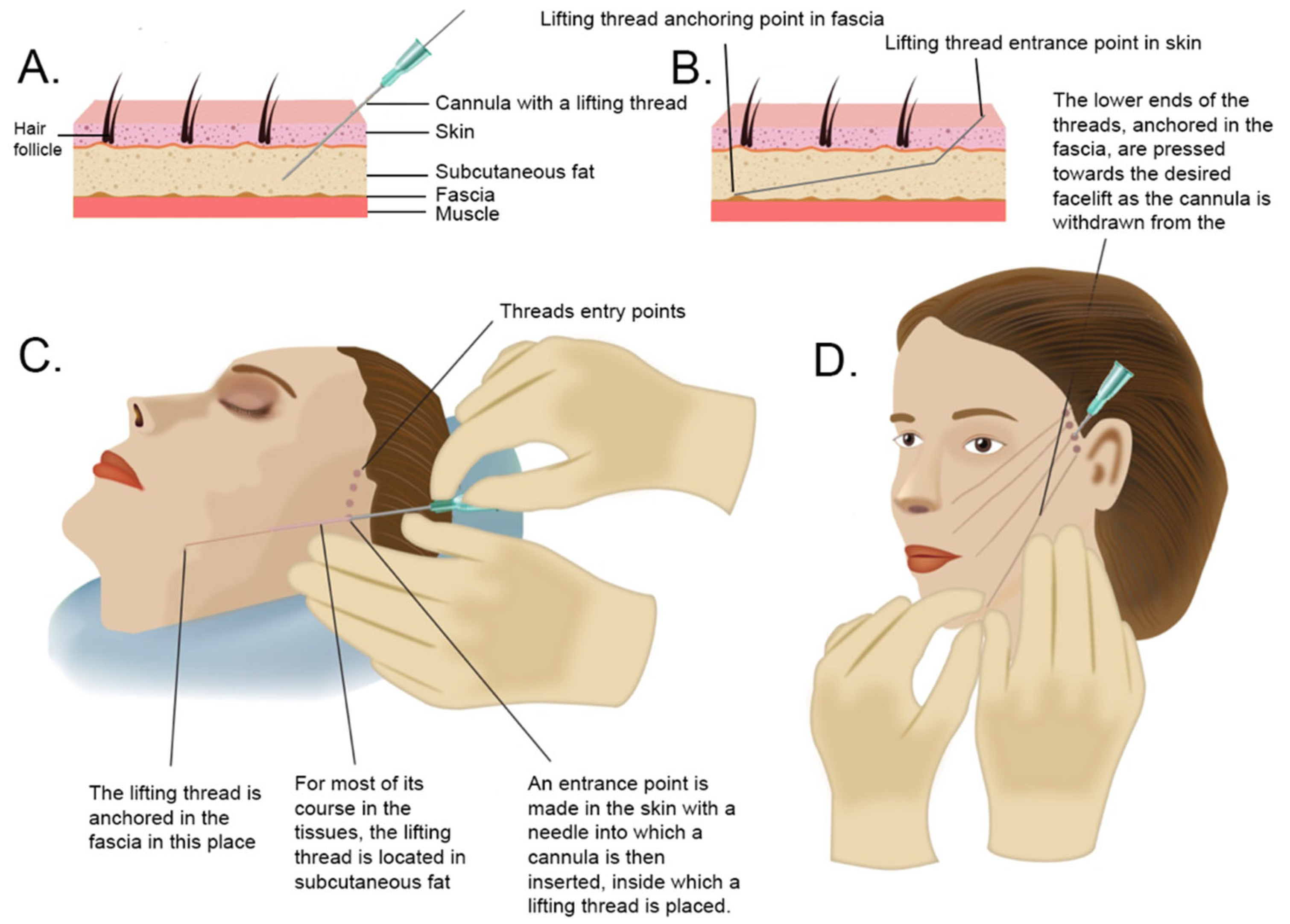
6. Lifting Threads
7. IPL and Laser Treatment
8. Mesotherapy
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanderpump, M.P.J. The epidemiology of thyroid disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef]
- Jacobson, E.M.; Tomer, Y. The genetic basis of thyroid autoimmunity. Thyroid 2007, 17, 949–961. [Google Scholar] [CrossRef]
- Hasham, A.; Tomer, Y. Genetic and epigenetic mechanisms in thyroid autoimmunity. Immunol. Res. 2012, 54, 204–213. [Google Scholar] [CrossRef]
- Tomer, Y.; Huber, A. The etiology of autoimmune thyroid disease: A story of genes and environment. J. Autoimmun. 2009, 32, 231–239. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Gough, S.C.L. Unravelling the genetic complexity of autoimmune thyroid disease: HLA, CTLA-4 and beyond. Clin. Exp. Immunol. 2004, 136, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, A.; Seaman, H.E.; Wright, J.W.; de Vries, C.S. The incidence of autoimmune thyroid disease: A systematic review of the literature. Clin. Endocrinol. 2008, 69, 687–696. [Google Scholar] [CrossRef]
- Stathatos, N.; Daniels, G.H. Autoimmune thyroid disease. Curr. Opin. Rheumatol. 2012, 24, 70–75. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Hegedüs, L.; Žarković, M.; Hickey, J.L.; Perros, P. Patient satisfaction and quality of life in hypothyroidism: An online survey by the british thyroid foundation. Clin. Endocrinol. 2021, 94, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Liping, D.; Lifen, Y.; Ruihong, D.; Zhihong, H. Analysis on the quality of life of the hyperthyroidism patients. In Proceedings of the 2011 International Conference on Human Health and Biomedical Engineering, Jilin, China, 19–22 August 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 791–794. [Google Scholar]
- Reitblat, O.; Lerman, T.T.; Reitblat, T. AB1349 Safety and effectiveness of cosmetic minimaly invasive procedures among patients with systemic autoimmune disease. Ann. Rheum. Dis. 2018, 77, 1763. [Google Scholar] [CrossRef]
- Coleman, S.R.; Grover, R. The anatomy of the aging face: Volume loss and changes in 3-dimensional topography. Aesthetic Surg. J. 2006, 26, S4–S9. [Google Scholar] [CrossRef]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef]
- Ozgentaş, H.E.; Pindur, A.; Spira, M.; Liu, B.; Shenaq, S. A comparison of soft-tissue substitutes. Ann. Plast. Surg. 1994, 33, 171–177. [Google Scholar] [CrossRef]
- Eppley, B.L.; Dadvand, B. Injectable soft-tissue fillers: Clinical overview. Plast. Reconstr. Surg. 2006, 118, 98e–106e. [Google Scholar] [CrossRef]
- American Society of Plastic Surgeons. National Plastic Surgery Statistics; American Society of Plastic Surgeons: Arlington Heights, IL, USA, 2020. [Google Scholar]
- Funt, D.; Pavicic, T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin. Cosmet. Investig. Dermatol. 2013, 6, 295–316. [Google Scholar] [CrossRef]
- Aljawhar, N.M.; Sharquie, I.K. Is hyaluronic acid filler still a potential risk factor for an autoimmune reaction? Med. J. Malaysia 2020, 75, 363–367. [Google Scholar]
- Melvin, O.G.; Hunt, K.M.; Jacobson, E.S. Hyaluronidase Treatment of Scleroderma-Induced Microstomia. JAMA Dermatol. 2019, 155, 857–859. [Google Scholar] [CrossRef]
- Watchmaker, J.; Saadeh, D.; Lam, C.; Vashi, N.A. A case of bilateral Parry-Romberg syndrome successfully treated with hyaluronic acid filler augmentation. J. Cosmet. Dermatol. 2019, 18, 1261–1263. [Google Scholar] [CrossRef]
- Ledon, J.A.; Savas, J.A.; Yang, S.; Franca, K.; Camacho, I.; Nouri, K. Inflammatory nodules following soft tissue filler use: A review of causative agents, pathology and treatment options. Am. J. Clin. Dermatol. 2013, 14, 401–411. [Google Scholar] [CrossRef]
- Requena, C.; Izquierdo, M.J.; Navarro, M.; Martínez, A.; Vilata, J.J.; Botella, R.; Amorrortu, J.; Sabater, V.; Aliaga, A.; Requena, L. Adverse reactions to injectable aesthetic microimplants. Am. J. Dermatopathol. 2001, 23, 197–202. [Google Scholar] [CrossRef]
- Sidwell, R.U.; Dhillon, A.P.; Butler, P.E.M.; Rustin, M.H.A. Localized granulomatous reaction to a semi-permanent hyaluronic acid and acrylic hydrogel cosmetic filler. Clin. Exp. Dermatol. 2004, 29, 630–632. [Google Scholar] [CrossRef]
- Ghislanzoni, M.; Bianchi, F.; Barbareschi, M.; Alessi, E. Cutaneous granulomatous reaction to injectable hyaluronic acid gel. Br. J. Dermatol. 2006, 154, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Yiannakopoulou, E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology 2015, 95, 65–69. [Google Scholar] [CrossRef]
- Gregoric, E.; Gregoric, J.A.; Guarneri, F.; Benvenga, S. Injections of Clostridium botulinum neurotoxin A may cause thyroid complications in predisposed persons based on molecular mimicry with thyroid autoantigens. Endocrine 2011, 39, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.J.; Vickers, S.F.; Lee, J.P. Botulinum toxin therapy in dysthyroid strabismus. Eye 1990, 4 Pt 4, 538–542. [Google Scholar] [CrossRef]
- Ozturk Karabulut, G.; Fazil, K.; Saracoglu Yilmaz, B.; Ozturker, C.; Günaydın, Z.K.; Taskapili, M.; Kaynak, P. An algorithm for Botulinum toxin A injection for upper eyelid retraction associated with thyroid eye disease: Long-term results. Orbit 2021, 40, 381–388. [Google Scholar] [CrossRef]
- Shih, M.-J.; Liao, S.-L.; Lu, H.-Y. A single transcutaneous injection with Botox for dysthyroid lid retraction. Eye 2004, 18, 466–469. [Google Scholar] [CrossRef]
- Costa, P.G.; Saraiva, F.P.; Pereira, I.C.; Monteiro, M.L.R.; Matayoshi, S. Comparative study of Botox injection treatment for upper eyelid retraction with 6-month follow-up in patients with thyroid eye disease in the congestive or fibrotic stage. Eye 2009, 23, 767–773. [Google Scholar] [CrossRef]
- Nava Castañeda, A.; Tovilla Canales, J.L.; Garnica Hayashi, L.; Velasco y Levy, A. Traitement de la rétraction palpébrale supérieure associée à l’ophtalmopathie dysthyroïdienne en phase active inflammatoire avec l’injection de toxine botulique A. J. Fr. Ophtalmol. 2017, 40, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhang, L.; Zhang, Y.-G. Does platelet-rich plasma enhance the survival of grafted fat? An update review. Int. J. Clin. Exp. Med. 2013, 6, 252–258. [Google Scholar]
- Mlynarek, R.A.; Kuhn, A.W.; Bedi, A. Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine. Am. J. Orthop. 2016, 45, 290–326. [Google Scholar]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin Therapy Lett. 2019, 24, 1–6. [Google Scholar]
- Justicz, N.; Derakhshan, A.; Chen, J.X.; Lee, L.N. Platelet-Rich Plasma for Hair Restoration. Facial Plast. Surg. Clin. N. Am. 2020, 28, 181–187. [Google Scholar] [CrossRef]
- Knop, E.; de Paula, L.E.; Fuller, R. Platelet-rich plasma for osteoarthritis treatment. Rev. Bras. Reumatol. 2016, 56, 152–164. [Google Scholar] [CrossRef]
- Hersant, B.; La Padula, S.; SidAhmed-Mezi, M.; Rodriguez, A.M.; Meningaud, J.P. Use of platelet-rich plasma (PRP) in microsurgery. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 236–237. [Google Scholar] [CrossRef]
- Feigin, K.; Shope, B. Use of Platelet-Rich Plasma and Platelet-Rich Fibrin in Dentistry and Oral Surgery: Introduction and Review of the Literature. J. Vet. Dent. 2019, 36, 109–123. [Google Scholar] [CrossRef]
- Cole, B.J.; Karas, V.; Hussey, K.; Pilz, K.; Fortier, L.A. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 339–346. [Google Scholar] [CrossRef]
- Tong, S.; Zhang, C.; Liu, J. Platelet-rich plasma exhibits beneficial effects for rheumatoid arthritis mice by suppressing inflammatory factors. Mol. Med. Rep. 2017, 16, 4082–4088. [Google Scholar] [CrossRef]
- Badsha, H.; Harifi, G.; Murrell, W.D. Platelet Rich Plasma for Treatment of Rheumatoid Arthritis: Case Series and Review of Literature. Case Rep. Rheumatol. 2020, 2020, 8761485. [Google Scholar] [CrossRef]
- Borhani-Haghighi, M.; Mohamadi, Y. The therapeutic effect of platelet-rich plasma on the experimental autoimmune encephalomyelitis mice. J. Neuroimmunol. 2019, 333, 476958. [Google Scholar] [CrossRef]
- Pabreja, V. Treatment of hypothyroidism with intra thyroidal injection of autologous platelet rich plasma and hyaluronic acid. In Proceedings of the 13th International Conference on Tissue Science, Engineering, Regenerative Medicine & Bio Banking, Vancouver, BC, Canada, 24–25 April 2019. [Google Scholar]
- Ralchenko, I.V.; Chepis, M.V.; Ralchenko, E.S. Thyroid pathology and platelet functional activity. In Proceedings of the Cmbebih 2017; Springer: Cham, Switzerland, 2017; pp. 344–349. [Google Scholar]
- Braverman, L.E.; Cooper, D.S.; Werner, S.C.; Ingbar, S.H. The Thyroid: A Fundamental and Clinical Text, 11th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2021. [Google Scholar]
- Lamberg, B.A.; Kivikangas, V.; Pelkonen, R.; Vuopio, P. Thrombocytopenia and decreased life-span of thrombocytes in hyperthyroidism. Ann. Clin. Res. 1971, 3, 98–102. [Google Scholar]
- Carlioglu, A.; Timur, O.; Durmaz, S.A.; Ayhan, M.E. Mean platelet volume in euthyroid patients with Hashimoto’s thyroiditis. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2015, 26, 282–284. [Google Scholar] [CrossRef]
- Ford, H.C.; Toomath, R.J.; Carter, J.M.; Delahunt, J.W.; Fagerstrom, J.N. Mean platelet volume is increased in hyperthyroidism. Am. J. Hematol. 1988, 27, 190–193. [Google Scholar] [CrossRef]
- Erikci, A.A.; Karagoz, B.; Ozturk, A.; Caglayan, S.; Ozisik, G.; Kaygusuz, I.; Ozata, M. The effect of subclinical hypothyroidism on platelet parameters. Hematology 2009, 14, 115–117. [Google Scholar] [CrossRef]
- Coban, E.; Yazicioglu, G.; Ozdogan, M. Platelet activation in subjects with subclinical hypothyroidism. Med. Sci. Monit. 2007, 13, CR211–CR214. [Google Scholar]
- Yilmaz, H.; Ertuğrul, O.; Ertuğrul, B.; Ertuğrul, D. Mean platelet volume in patients with subclinical hypothyroidism. Platelets 2011, 22, 143–147. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.H.; Kim, S.Y.; Bae, H.Y. The mean platelet volume is positively correlated with serum thyrotropin concentrations in a population of healthy subjects and subjects with unsuspected subclinical hypothyroidism. Thyroid 2013, 23, 31–37. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Schulz, A.; Hermanns, M.I.; Grossmann, V.; Pefani, E.; Spronk, H.M.H.; Laubert-Reh, D.; Binder, H.; Beutel, M.; Pfeiffer, N.; et al. Sex-specific differences in genetic and nongenetic determinants of mean platelet volume: Results from the Gutenberg Health Study. Blood 2016, 127, 251–259. [Google Scholar] [CrossRef]
- Ijaz, S.H.; Jamal, S.M.; Qayyum, R. Relationship Between Thyroid Hormone Levels and Mean Platelet Count and Volume: Quantitative Assessment. Cureus 2018, 10, e3421. [Google Scholar] [CrossRef]
- Masunaga, R.; Nagasaka, A.; Nakai, A.; Kotake, M.; Sawai, Y.; Oda, N.; Mokuno, T.; Shimazaki, K.; Hayakawa, N.; Kato, R.; et al. Alteration of platelet aggregation in patients with thyroid disorders. Metabolism. 1997, 46, 1128–1131. [Google Scholar] [CrossRef]
- Coleman, S.R. Long-Term Survival of Fat Transplants: Controlled Demonstrations. Aesthetic Plast. Surg. 2020, 44, 1268–1272. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural fat grafting: More than a permanent filler. Plast. Reconstr. Surg. 2006, 118, 108S–120S. [Google Scholar] [CrossRef]
- Bellini, E.; Grieco, M.P.; Raposio, E. A journey through liposuction and liposculture: Review. Ann. Med. Surg. 2017, 24, 53–60. [Google Scholar] [CrossRef]
- Klein, J.A. The tumescent technique. Anesthesia and modified liposuction technique. Dermatol. Clin. 1990, 8, 425–437. [Google Scholar] [CrossRef]
- Kling, R.E.; Mehrara, B.J.; Pusic, A.L.; Young, V.L.; Hume, K.M.; Crotty, C.A.; Rubin, J.P. Trends in autologous fat grafting to the breast: A national survey of the american society of plastic surgeons. Plast. Reconstr. Surg. 2013, 132, 35–46. [Google Scholar] [CrossRef]
- Trojahn Kølle, S.-F.; Oliveri, R.S.; Glovinski, P.V.; Elberg, J.J.; Fischer-Nielsen, A.; Drzewiecki, K.T. Importance of mesenchymal stem cells in autologous fat grafting: A systematic review of existing studies. J. Plast. Surg. Hand Surg. 2012, 46, 59–68. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural fat grafts: The ideal filler? Clin. Plast. Surg. 2001, 28, 111–119. [Google Scholar] [CrossRef]
- Magalon, G.; Daumas, A.; Sautereau, N.; Magalon, J.; Sabatier, F.; Granel, B. Regenerative Approach to Scleroderma with Fat Grafting. Clin. Plast. Surg. 2015, 42, 353–364. [Google Scholar] [CrossRef]
- Gheisari, M.; Ahmadzadeh, A.; Nobari, N.; Iranmanesh, B.; Mozafari, N. Autologous Fat Grafting in the Treatment of Facial Scleroderma. Dermatol. Res. Pract. 2018, 2018, 6568016. [Google Scholar] [CrossRef]
- Daumas, A.; Magalon, J.; Delaunay, F.; Abellan, M.; Philandrianos, C.; Sabatier, F.; Granel, B.; Magalon, G. Fat Grafting for Treatment of Facial Scleroderma. Clin. Plast. Surg. 2020, 47, 155–163. [Google Scholar] [CrossRef]
- Del Papa, N.; Di Luca, G.; Andracco, R.; Zaccara, E.; Maglione, W.; Pignataro, F.; Minniti, A.; Vitali, C. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: Results of a monocentric randomized controlled study. Arthritis Res. Ther. 2019, 21, 7. [Google Scholar] [CrossRef]
- Oberlin, D.; Lynam, K.; Douglass, M. Granulomatous reaction to autologous gluteal fat transfer. JAAD Case Rep. 2019, 5, 522–524. [Google Scholar] [CrossRef][Green Version]
- Rapkiewicz, A.V.; Kenerson, K.; Hutchins, K.D.; Garavan, F.; Lew, E.O.; Shuman, M.J. Fatal Complications of Aesthetic Techniques: The Gluteal Region. J. Forensic Sci. 2018, 63, 1406–1412. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Long, X.; Zhang, M.; Huang, J.; Yu, N.; Xu, J. Supportive Use of Adipose-Derived Stem Cells in Cell-Assisted Lipotransfer for Localized Scleroderma. Plast. Reconstr. Surg. 2018, 141, 1395–1407. [Google Scholar] [CrossRef]
- Mineda, K.; Kuno, S.; Kato, H.; Kinoshita, K.; Doi, K.; Hashimoto, I.; Nakanishi, H.; Yoshimura, K. Chronic inflammation and progressive calcification as a result of fat necrosis: The worst outcome in fat grafting. Plast. Reconstr. Surg. 2014, 133, 1064–1072. [Google Scholar] [CrossRef]
- Pucciarelli, V.; Baserga, C.; Codari, M.; Beltramini, G.A.; Sforza, C.; Giannì, A.B. Three-Dimensional Stereophotogrammetric Evaluation of the Efficacy of Autologous Fat Grafting in the Treatment of Parry-Romberg Syndrome. J. Craniofac. Surg. 2018, 29, 2124–2127. [Google Scholar] [CrossRef]
- Gierloff, M.; Seeck, N.G.K.; Springer, I.; Becker, S.; Kandzia, C.; Wiltfang, J. Orbital floor reconstruction with resorbable polydioxanone implants. J. Craniofac. Surg. 2012, 23, 161–164. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Sulamanidze, M.; Sulamanidze, G. APTOS suture lifting methods: 10 years of experience. Clin. Plast. Surg. 2009, 36, 281–306. [Google Scholar] [CrossRef]
- Abraham, R.F.; DeFatta, R.J.; Williams, E.F. 3rd Thread-lift for facial rejuvenation: Assessment of long-term results. Arch. Facial Plast. Surg. 2009, 11, 178–183. [Google Scholar] [CrossRef]
- Sapountzis, S.; Kim, J.H.; Li, T.-S.; Rashid, A.; Cruz, P.C.; Hwang, Y.S. Successful treatment of thread-lifting complication from APTOS sutures using a simple MACS lift and fat grafting. Aesthetic Plast. Surg. 2012, 36, 1307–1310. [Google Scholar] [CrossRef]
- Goldan, O.; Bank, J.; Regev, E.; Haik, J.; Winkler, E. Epidermoid inclusion cysts After APTOS thread insertion: Case report with clinicopathologic correlates. Aesthetic Plast. Surg. 2008, 32, 147–148. [Google Scholar] [CrossRef]
- Winkler, E.; Goldan, O.; Regev, E.; Mendes, D.; Orenstein, A.; Haik, J. Stensen duct rupture (sialocele) and other complications of the Aptos thread technique. Plast. Reconstr. Surg. 2006, 118, 1468–1471. [Google Scholar] [CrossRef]
- Sarigul Guduk, S.; Karaca, N. Safety and complications of absorbable threads made of poly-L-lactic acid and poly lactide/glycolide: Experience with 148 consecutive patients. J. Cosmet. Dermatol. 2018, 17, 1189–1193. [Google Scholar] [CrossRef]
- Aptos LLC. [Internet]. The Indications and Contraindications for a Thread Lifting. Available online: https://aptos.global/archive/30/the-indications-and-contraindications-for-a-thread-lifting (accessed on 5 November 2021).
- Sakai, T.; Kohno, H.; Ishihara, T.; Higaki, M.; Saito, S.; Matsushima, M.; Mizushima, Y.; Kitahara, K. Treatment of experimental autoimmune uveoretinitis with poly(lactic acid) nanoparticles encapsulating betamethasone phosphate. Exp. Eye Res. 2006, 82, 657–663. [Google Scholar] [CrossRef]
- Gollapudi, S. Safety and Efficacy of Polydioxanone Nano-Fibers as Anti-Inflammatory Agents. J. Nanomedine. Biotherapeutic Discov. 2014, 4, 1000127. [Google Scholar] [CrossRef]
- Titley-Diaz, W.H.; de Cicco, F.L. Suture Hypersensitivity; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562288/ (accessed on 5 November 2021).
- Höfling, D.B.; Chavantes, M.C.; Buchpiguel, C.A.; Cerri, G.G.; Marui, S.; Carneiro, P.C.; Chammas, M.C. Safety and Efficacy of Low-Level Laser Therapy in Autoimmune Thyroiditis: Long-Term Follow-Up Study. Int. J. Endocrinol. 2018, 2018, 8387530. [Google Scholar] [CrossRef]
- Lee, Y.I.; Lee, E.; Nam, K.-H.; Shin, D.Y.; Kim, J.; Suk, J.; Kwak, J.Y.; Lee, J.H. The Use of a Light-Emitting Diode Device for Neck Rejuvenation and Its Safety on Thyroid Glands. J. Clin. Med. 2021, 10, 1774. [Google Scholar] [CrossRef]
- Wickenheisser, V.A.; Zywot, E.M.; Rabjohns, E.M.; Lee, H.H.; Lawrence, D.S.; Tarrant, T.K. Laser Light Therapy in Inflammatory, Musculoskeletal, and Autoimmune Disease. Curr. Allergy Asthma Rep. 2019, 19, 37. [Google Scholar] [CrossRef]
- Byun, Y.S.; Son, J.H.; Cho, Y.S.; Chung, B.Y.; Cho, H.J.; Park, C.W.; Kim, H.O. Intense Pulsed Light and Q-Switched 1,064-nm Neodymium-Doped Yttrium Aluminum Garnet Laser Treatment for the Scarring Lesion of Discoid Lupus Erythematosus. Ann. Dermatol. 2017, 29, 331–333. [Google Scholar] [CrossRef]
- Ekbäck, M.P.; Troilius, A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J. Cosmet. Laser Ther. 2013, 15, 260–265. [Google Scholar] [CrossRef]
- Levy, J.L. Intense pulsed light treatment for chronic facial erythema of systemic lupus erythematosus: A case report. J. Cutan. Laser Ther. 2000, 2, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Erceg, A.; Bovenschen, H.J.; van de Kerkhof, P.C.M.; de Jong, E.M.J.G.; Seyger, M.M.B. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J. Am. Acad. Dermatol. 2009, 60, 626–632. [Google Scholar] [CrossRef]
- Núñez, M.; Boixeda, P.; Miralles, E.S.; de Misa, R.F.; Ledo, A. Pulsed Dye Laser Treatment of Telangiectatic Chronic Erythema of Cutaneous Lupus Erythematosus. Arch. Dermatol. 1996, 132, 354–355. [Google Scholar] [CrossRef]
- Maushagen-Schnaas, E.; Raulin, C. Lupus erythematodes: Ttherapie von kutanen Läsionen mit dem gepulsten Farbstofflaser. Aktuelle Derm. 1997, 23, 173–176. [Google Scholar]
- Raulin, C.; Schmidt, C.; Hellwig, S. Cutaneous lupus erythematosus-treatment with pulsed dye laser. Br. J. Dermatol. 1999, 141, 1046–1050. [Google Scholar] [CrossRef]
- Kim, J.-T.; Choi, A.; Jeong, J.-H.; Jo, J.-H.; Ryu, O.-S.; Kim, E.-J.; Kim, K.-Y.; Song, M.-H.; Song, Y.-H.; Shin, W.-S.; et al. Safety evaluation and consideration of 4 Pin Multi-needle for meso-therapy. Technol. Heal. Care 2018, 26, 291–306. [Google Scholar] [CrossRef]
- Markiewicz, A.; Zasada, M.; Erkiert-Polguj, A.; Wieckowska-Szakiel, M.; Budzisz, E. An evaluation of the antiaging properties of strawberry hydrolysate treatment enriched with L-ascorbic acid applied with microneedle mesotherapy. J. Cosmet. Dermatol. 2019, 18, 129–135. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jeong, K.H.; Kim, J.E.; Woo, Y.J.; Kim, B.J.; Kang, H. Repeated Microneedle Stimulation Induces Enhanced Hair Growth in a Murine Model. Ann. Dermatol. 2016, 28, 586–592. [Google Scholar] [CrossRef]
- Rittes, P.G. The use of phosphatidylcholine for correction of localized fat deposits. Aesthetic Plast. Surg. 2003, 27, 315–318. [Google Scholar] [CrossRef]
- Rotunda, A.M.; Suzuki, H.; Moy, R.L.; Kolodney, M.S. Detergent effects of sodium deoxycholate are a major feature of an injectable phosphatidylcholine formulation used for localized fat dissolution. Dermatologic Surg. 2004, 30, 1001–1008. [Google Scholar] [CrossRef]
- Duncan, D.I.; Hasengschwandtner, F. Lipodissolve for subcutaneous fat reduction and skin retraction. Aesthetic Surg. J. 2005, 25, 530–543. [Google Scholar] [CrossRef][Green Version]
- Young, V.L. Lipostabil: The effect of phosphatidylcholine on subcutaneous fat. Aesthetic Surg. J. 2003, 23, 413–417. [Google Scholar] [CrossRef][Green Version]
- Marrif, H.I. Thyroid hormones and mesotherapy. Front. Endocrinol. 2011, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Danilovic, D.L.S.; Bloise, W.; Knobel, M.; Marui, S. Factitious thyrotoxicosis induced by mesotherapy: A case report. Thyroid 2008, 18, 655–657. [Google Scholar] [CrossRef]
- BS, C. Triamcinolone Acetonide Mesotherapy in the Treatment of Recalcitrant Patches of Alopecia Areata—A Pilot Study. Clin. Dermatology Ther. 2015, 2, 1–4. [Google Scholar] [CrossRef]
- Kandhari, R.; Kaur, I.; Sharma, D. Mesococktails and mesoproducts in aesthetic dermatology. Dermatol. Ther. 2020, 33, e14218. [Google Scholar] [CrossRef]
- De Carvalho, G.A.; Ramos, H.E. Thyroid hormone resistance syndrome. Arq. Bras. Endocrinol. Metabol. 2004, 48, 83–92. [Google Scholar] [CrossRef][Green Version]
- Colón-Soto, M.; Peredo, R.A.; Vilá, L.M. Systemic lupus erythematosus after mesotherapy with acetyl-L-carnitine. J. Clin. Rheumatol. 2006, 12, 261–262. [Google Scholar] [CrossRef]

| Procedure Type | Procedure Indications | Safety and Efficacy in Autoimmune Thyroiditis |
|---|---|---|
| Autologous fat grafting | Tissue volume restoration, facial contouring, facial asymmetry, scars, burns, radiation dermatitis, HIV-associated lipodystrophy, breast augmentation, buttock augmentation | Safe; however, graft survival may be reduced |
| Autologous platelet-rich plasma | Androgenic alopecia, acne scars, dermal augmentation (facial rhytides), therapies combined with fractional laser (resurfacing), autologous fat grafting (tissue augmentation), radio frequency (skin density) | Safe; however, efficacy may be reduced due to platelets disfunction, especially in hyperthyroidism. |
| Botulinum toxin | Wrinkle correction, strabismus, blepharospasm, chronic migraine | Safe and efficient |
| Hyaluronic acid | Tissue volume restoration, facial contouring, wrinkle correction, nonsurgical rhinoplasty | Relatively safe; however, inflammatory nodes may occur |
| IPL and laser treatment | Skin resurfacing, non-muscular wrinkles, telangiectatic naevi, acne rosacea, radiodermatitis, erythrosis of the neck, Ota’s nevus, Becker’s nevus, liver spots, eccrine angiomatous hamartoma, dermo-epidermal lesions | Safe and efficient |
| Lifting threads | face wrinkles, forehead lift, face lift | Unknown safety, procedure not recommended |
| Mesotherapy | Face wrinkles, insufficiently hydrated and nourished skin- skin rejuvenation, skin tightening, photoaging, alopecia, cellulite, local fat deposits, hyperpigmentation and melasma, telangiectasias, vitiligo, eczema | Unknown safety, procedure not recommended |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, K.; Rusyan, E.; Franek, E. Safety of Aesthetic Medicine Procedures in Patients with Autoimmune Thyroid Disease: A Literature Review. Medicina 2022, 58, 30. https://doi.org/10.3390/medicina58010030
Adamczyk K, Rusyan E, Franek E. Safety of Aesthetic Medicine Procedures in Patients with Autoimmune Thyroid Disease: A Literature Review. Medicina. 2022; 58(1):30. https://doi.org/10.3390/medicina58010030
Chicago/Turabian StyleAdamczyk, Kamil, Ewa Rusyan, and Edward Franek. 2022. "Safety of Aesthetic Medicine Procedures in Patients with Autoimmune Thyroid Disease: A Literature Review" Medicina 58, no. 1: 30. https://doi.org/10.3390/medicina58010030
APA StyleAdamczyk, K., Rusyan, E., & Franek, E. (2022). Safety of Aesthetic Medicine Procedures in Patients with Autoimmune Thyroid Disease: A Literature Review. Medicina, 58(1), 30. https://doi.org/10.3390/medicina58010030





