The Immunohistochemical Expression of the Serine and Arginine-Rich Splicing Factor 1 (SRSF1) Is a Predictive Factor of the Recurrence of Basal Cell Carcinoma: A Preliminary Study on a Series of 52 Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Sample Collection
2.2. Immunohistochemistry
3. Results
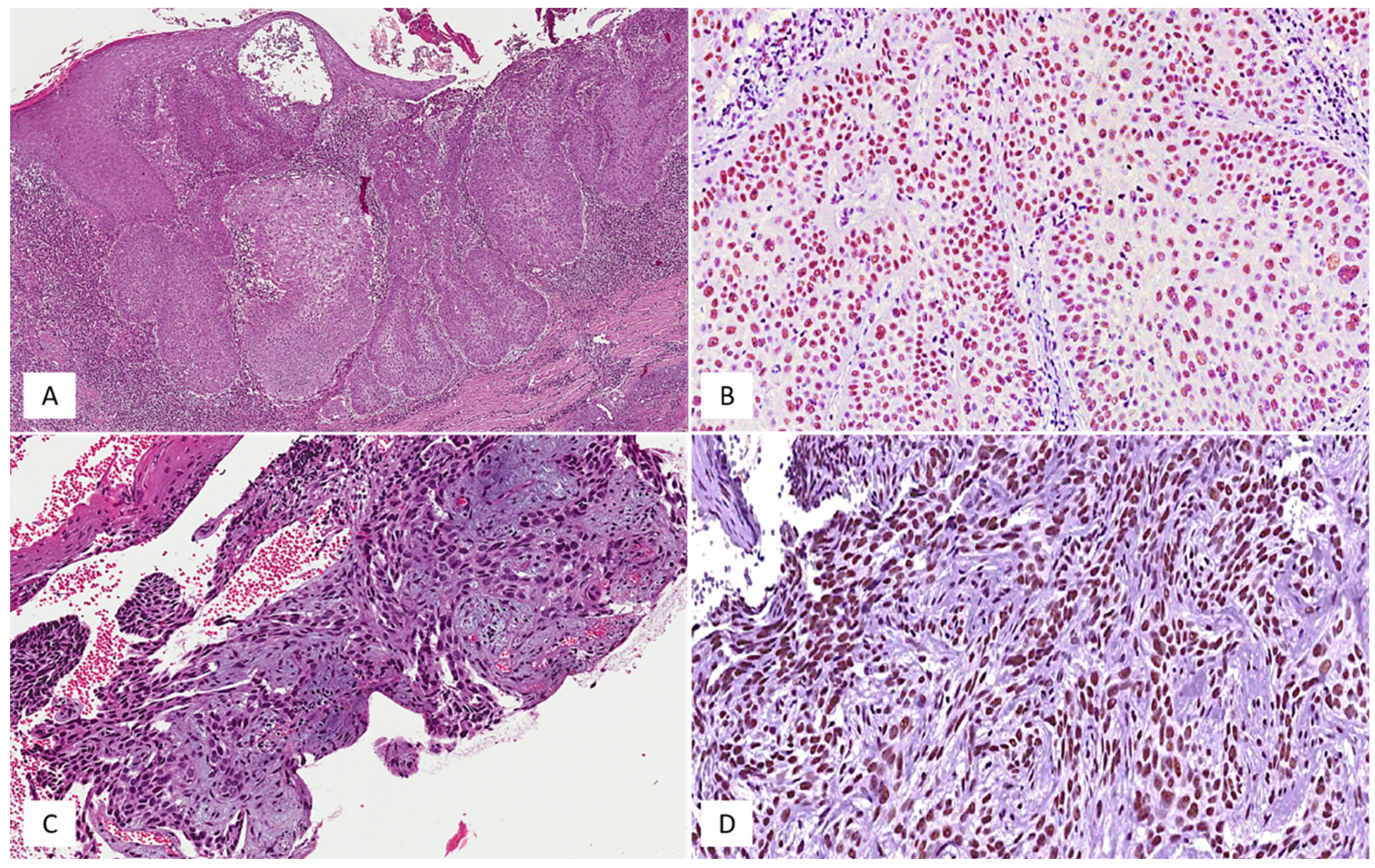
3.1. Clinicopathologic Features of the BCC Cases from Our Series
3.2. SRSF1’s Immunohistochemical Expression and Its Relationship with the Local Recurrence of BCCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verkouteren, J.; Ramdas, K.; Wakkee, M.; Nijsten, T. Epidemiology of basal cell carcinoma: Scholarly review. Br. J. Dermatol. 2017, 177, 359–372. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus–based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Broggi, G.; Lacarrubba, F.; Verzì, A.E.; Micali, G.; Caltabiano, R. Confocal microscopy features of patch-stage mycosis fungoides and their correlation with horizontal histopathological sections. A case series. J. Cutan. Pathol. 2019, 46, 163–165. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Verzì, A.E.; Caltabiano, R.; Broggi, G.; Di Natale, A.; Micali, G. Discoid lupus erythematosus: Reflectance confocal microscopy features correlate with horizontal histopathological sections. Ski. Res. Technol. 2019, 25, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Verzì, A.E.; Lacarrubba, F.; Caltabiano, R.; Broggi, G.; Musumeci, M.L.; Micali, G. Reflectance Confocal Microscopy Features of Plaque Psoriasis Overlap with Horizontal Histopathological Sections: A Case Series. Am. J. Dermatopathol. 2019, 41, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-Field Confocal Optical Coherence Tomography May Enhance Monitoring of Superficial Basal Cell Carcinoma Treated with Imiquimod 5% Cream: A Pilot Study. Cancers 2021, 13, 4913. [Google Scholar] [CrossRef]
- Broggi, G.; Verzì, A.E.; Caltabiano, R.; Micali, G.; Lacarrubba, F. Correlation Between In Vivo Reflectance Confocal Microscopy and Horizontal Histopathology in Skin Cancer: A Review. Front. Oncol. 2021, 11, 653140. [Google Scholar] [CrossRef]
- Marinho, S.A.; Lima, N.L.; Verli, F.D.; De Miranda, J.L. Basosquamous carcinoma: Histopathological features. Indian J. Dermatol. 2012, 57, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Trakatelli, M.; Morton, C.C.; Nagore, E.; Ulrich, C.; Del Marmol, V.; Peris, K.; Basset-Seguin, N.N. Update of the European guidelines for basal cell carcinoma management. Eur. J. Dermatol. 2014, 24, 312–329. [Google Scholar] [CrossRef]
- Lara, F.; Santamaría, J.R.; Garbers, L.E.F.D.M.; Paraná, B.F.E.D. Recurrence rate of basal cell carcinoma with positive histopathological margins and related risk factors. An. Bras. Dermatol. 2017, 92, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Vornicescu, C.; Șenilă, S.C.; Bejinariu, N.I.; Vesa, Ș.C.; Boșca, A.B.; Chirilă, D.N.; Melincovici, C.S.; Sorițău, O.; Mihu, C.M. Predictive factors for the recurrence of surgically excised basal cell carcinomas: A retrospective clinical and immunopathological pilot study. Exp. Ther. Med. 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, F.; Li, Z.; Xie, Y.; Yu, X. RNA binding protein serine/arginine splicing factor 1 promotes the proliferation, migration and invasion of hepatocellular carcinoma by interacting with RecQ protein-like 4 mRNA. Bioengineered 2021, 12, 6144–6154. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-W.; Xu, D.; Chen, W.-J.; Chen, J.-X.; Chen, W.-J.; Ye, J.-Q.; Gan, S.-S.; Zhou, W.; Song, X.; Shi, L.; et al. USP39 promotes malignant proliferation and angiogenesis of renal cell carcinoma by inhibiting VEGF-A165b alternative splicing via regulating SRSF1 and SRPK1. Cancer Cell Int. 2021, 21, 1–16. [Google Scholar] [CrossRef]
- Barbagallo, D.; Caponnetto, A.; Barbagallo, C.; Battaglia, R.; Mirabella, F.; Brex, D.; Stella, M.; Broggi, G.; Altieri, R.; Certo, F.; et al. The GAUGAA Motif Is Responsible for the Binding between circSMARCA5 and SRSF1 and Related Downstream Effects on Glioblastoma Multiforme Cell Migration and Angiogenic Potential. Int. J. Mol. Sci. 2021, 22, 1678. [Google Scholar] [CrossRef]
- Du, J.-X.; Luo, Y.-H.; Zhang, S.-J.; Wang, B.; Chen, C.; Zhu, G.-Q.; Zhu, P.; Cai, C.-Z.; Wan, J.-L.; Cai, J.-L.; et al. Splicing factor SRSF1 promotes breast cancer progression via oncogenic splice switching of PTPMT1. J. Exp. Clin. Cancer Res. 2021, 40, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Laliotis, G.I.; Chavdoula, E.; Paraskevopoulou, M.D.; Kaba, A.; La Ferlita, A.; Singh, S.; Anastas, V.; Nair, K.A.; Orlacchio, A.; Taraslia, V.; et al. AKT3-mediated IWS1 phosphorylation promotes the proliferation of EGFR-mutant lung adenocarcinomas through cell cycle-regulated U2AF2 RNA splicing. Nat. Commun. 2021, 12, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Broggi, G.; Salvatorelli, L.; Barbagallo, D.; Certo, F.; Altieri, R.; Tirrò, E.; Massimino, M.; Vigneri, P.; Guadagno, E.; Maugeri, G.; et al. Diagnostic Utility of the Immunohistochemical Expression of Serine and Arginine Rich Splicing Factor 1 (SRSF1) in the Differential Diagnosis of Adult Gliomas. Cancers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Broggi, G.; Giudice, A.L.; Di Mauro, M.; Asmundo, M.G.; Pricoco, E.; Piombino, E.; Caltabiano, R.; Morgia, G.; Russo, G.I. SRSF-1 and microvessel density immunohistochemical analysis by semi-automated tissue microarray in prostate cancer patients with diabetes (DIAMOND study). Prostate 2021, 81, 882–892. [Google Scholar] [CrossRef]
- Russo, D.; Di Crescenzo, R.M.; Broggi, G.; Merolla, F.; Martino, F.; Varricchio, S.; Ilardi, G.; Borzillo, A.; Carandente, R.; Pignatiello, S.; et al. Expression of P16INK4a in Uveal Melanoma: New Perspectives. Front. Oncol. 2020, 10, 562074. [Google Scholar] [CrossRef] [PubMed]
- Bøgelund, F.; Philipsen, P.; Gniadecki, R. Factors Affecting the Recurrence Rate of Basal Cell Carcinoma. Acta Derm. Venereol. 2007, 87, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, A.; Zhang, X.; Wang, X.; Zhang, J.; Ma, J. lncRNA LINC01296 Promotes Oral Squamous Cell Carcinoma Development by Binding with SRSF1. BioMed Res. Int. 2021, 2021, 6661520. [Google Scholar] [CrossRef]
- Duan, Y.; Jia, Y.; Wang, J.; Liu, T.; Cheng, Z.; Sang, M.; Lv, W.; Qin, J.; Liu, L. Long noncoding RNA DGCR5 involves in tumorigenesis of esophageal squamous cell carcinoma via SRSF1-mediated alternative splicing of Mcl-1. Cell Death Dis. 2021, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zhang, B.; Huang, L.; Zheng, Z.; Xie, S.; Shen, L.; Breitzig, M.; Czachor, A.; Liu, H.; Luo, H.; et al. SRSF1 promotes the inclusion of exon 3 of SRA1 and the invasion of hepatocellular carcinoma cells by interacting with exon 3 of SRA1pre-mRNA. Cell Death Discov. 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Pan, D. The Hippo Signaling Pathway in Development and Cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [Green Version]
- Szelachowska, J.; Donizy, P.; Ratajczak-Wielgomas, K.; Halon, A.; Zielecka-Debska, D.; Lichon, K.; Maciejczyk, A.; Lata-Wozniak, E.; Piotrowska, A.; Matkowski, R. The effect of YAP expression in tumor cells and tumor stroma on the prognosis of patients with squamous cell carcinoma of the oral cavity floor and oral surface of the tongue. Oncol. Lett. 2019, 18, 3561–3570. [Google Scholar] [CrossRef] [Green Version]
- Mole, S.; Faizo, A.; Hernandez-Lopez, H.; Griffiths, M.; Stevenson, A.; Roberts, S.; Graham, S.V. Human papillomavirus type 16 infection activates the host serine arginine protein kinase 1 (SRPK1)—Splicing factor axis. J. Gen. Virol. 2020, 101, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, K.; Regalbuto, E.; Pierucci, F.; Arvia, R.; Mazzoli, S.; Gori, A.; De Giorgi, V. Pattern of HPV infection in basal cell carcinoma and in perilesional skin biopsies from immunocompetent patients. Virol. J. 2012, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, M.; Sadeghi, M. Human papilloma virus infection in basal cell carcinoma of the skin: A systematic review and meta-analysis study. Pol. J. Pathol. 2017, 68, 330–342. [Google Scholar] [CrossRef] [Green Version]

| Number of Cases | Age Range | Gender | Anatomic Site | Local Recurrence | Histological Subtype | SRSF1 IRS |
|---|---|---|---|---|---|---|
| 52 | 36–84 y | 32 M; 20 F | Arms (n = 21) Legs (n = 15) Face (n = 10) Shoulders (n = 5) Ankles (n = 1) | No (n = 47) Yes (n = 5) | Nodular BCC (n = 27) Superficial BCC (n = 15) Sclerosing/morphoeic BCC (n = 6) Basosquamous carcinoma (n = 4) | L-IRS (n = 34) H-IRS (n = 18) |
| Local Recurrence | High SRSF1 IRS | Low SRSF1 IRS |
|---|---|---|
| No | 14 | 33 |
| Yes | 4 | 1 |
| Gender | High SRSF1 IRS | Low SRSF1 IRS |
|---|---|---|
| Female | 6 | 14 |
| Male | 12 | 20 |
| Anatomic Site | High SRSF1 IRS | Low SRSF1 IRS |
|---|---|---|
| Ankles | 1 | 0 |
| Arms | 4 | 17 |
| Face | 5 | 5 |
| Legs | 4 | 11 |
| Shoulders | 4 | 1 |
| Histological Subtype | High SRSF1 IRS | Low SRSF1 IRS |
|---|---|---|
| Basosquamous | 2 | 2 |
| Nodular | 5 | 22 |
| Sclerosing/morphoeic | 2 | 4 |
| Superficial | 9 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broggi, G.; Barbagallo, D.; Lacarrubba, F.; Verzì, A.E.; Micali, G.; Purrello, M.; Caltabiano, R. The Immunohistochemical Expression of the Serine and Arginine-Rich Splicing Factor 1 (SRSF1) Is a Predictive Factor of the Recurrence of Basal Cell Carcinoma: A Preliminary Study on a Series of 52 Cases. Medicina 2022, 58, 139. https://doi.org/10.3390/medicina58010139
Broggi G, Barbagallo D, Lacarrubba F, Verzì AE, Micali G, Purrello M, Caltabiano R. The Immunohistochemical Expression of the Serine and Arginine-Rich Splicing Factor 1 (SRSF1) Is a Predictive Factor of the Recurrence of Basal Cell Carcinoma: A Preliminary Study on a Series of 52 Cases. Medicina. 2022; 58(1):139. https://doi.org/10.3390/medicina58010139
Chicago/Turabian StyleBroggi, Giuseppe, Davide Barbagallo, Francesco Lacarrubba, Anna Elisa Verzì, Giuseppe Micali, Michele Purrello, and Rosario Caltabiano. 2022. "The Immunohistochemical Expression of the Serine and Arginine-Rich Splicing Factor 1 (SRSF1) Is a Predictive Factor of the Recurrence of Basal Cell Carcinoma: A Preliminary Study on a Series of 52 Cases" Medicina 58, no. 1: 139. https://doi.org/10.3390/medicina58010139
APA StyleBroggi, G., Barbagallo, D., Lacarrubba, F., Verzì, A. E., Micali, G., Purrello, M., & Caltabiano, R. (2022). The Immunohistochemical Expression of the Serine and Arginine-Rich Splicing Factor 1 (SRSF1) Is a Predictive Factor of the Recurrence of Basal Cell Carcinoma: A Preliminary Study on a Series of 52 Cases. Medicina, 58(1), 139. https://doi.org/10.3390/medicina58010139









