Selective IgA Deficiency and Allergy: A Fresh Look to an Old Story
Abstract
1. Introduction
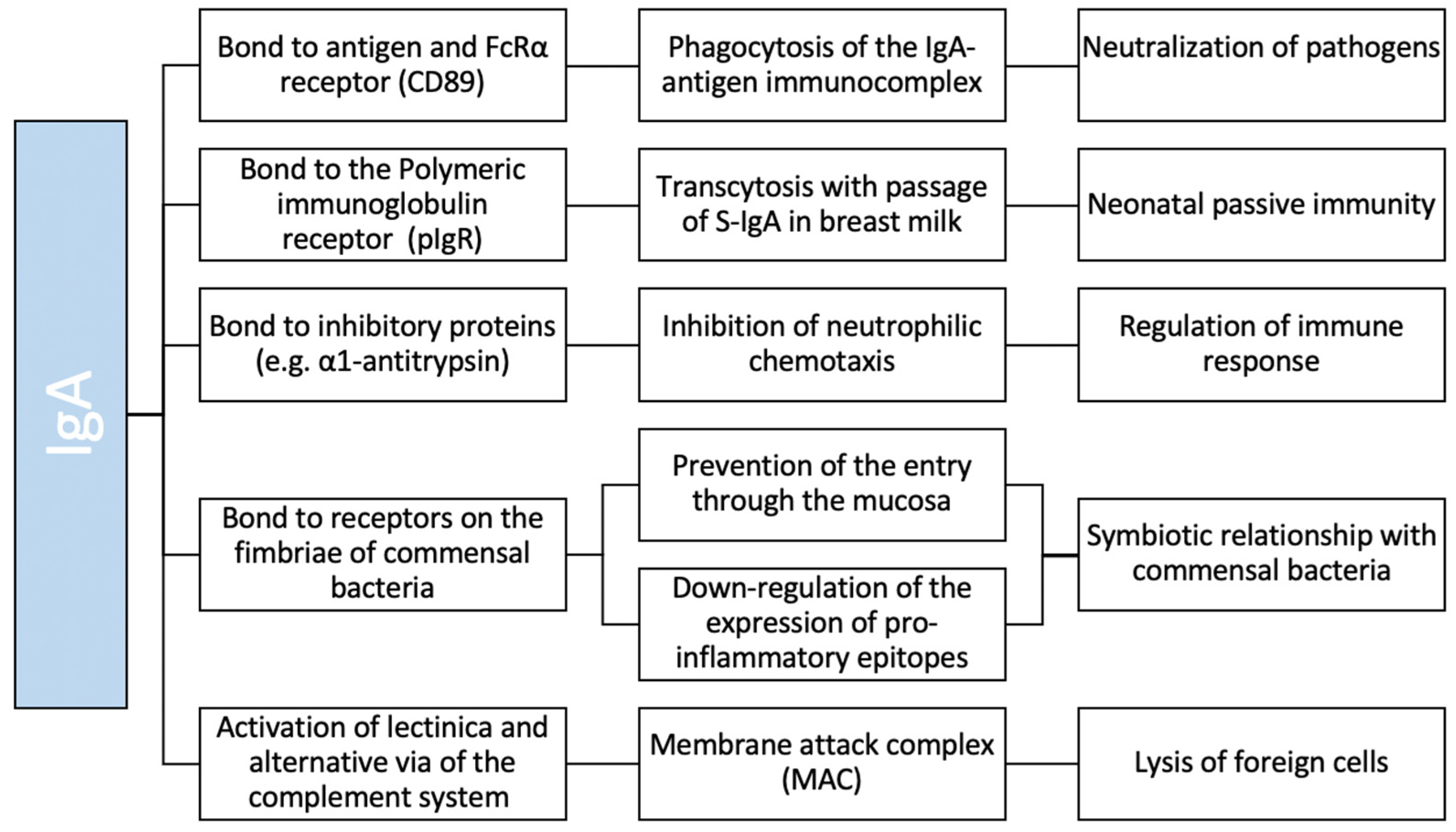
2. IgA Structure and Function
3. Selective IgA Deficiency (SIgAD)
3.1. Definition and Incidence
3.2. SIgAD Pathogenesis
3.3. Inheritance and Genetics
3.4. Clinical Manifestations
4. SIgAD and Allergy
4.1. Atopic Dermatitis (AD)
4.2. Food Allergy
4.3. Asthma and Recurrent Infections
| Authors, Year (References) | Country | N of pts | Type of pts (P/A) | Allergy, n (%) | Controls Included | Asthma, n (%) | Rhinitis, n (%) | Atopic Dermatitis, n (%) | Urticaria, n (%) | Food Allergy, n (%) | Sensitisation, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Janzi, 2009 [62] | Sweden | 14 | P | 4 (29) | Yes | 1 (7) | NA | 3 (21) | NA | 4 (29) | 4 (29) |
| Aghamohammadi, 2009 [7] | Iran | 37 | P + A | 31 (84) | No | 19 (51) | 16 (43) | 18 (49) | NA | 8 (22) | 31 (48) |
| Shkalim, 2010 [91] | Israel | 63 | P | 20 (32) | No | 15 (24) | 8 (13) | 2 (3.2) | 2 (3) | NA | NA |
| Erkocoğlu, 2017 [69] | Turkey | 81 | P | 37 (46) | No | 28 (35) | 22 (27) | 9 (11.1) | NA | 1 (1) | 18 (22) |
| Aytekin, 2012 [65] | Turkey | 118 | P | 51 (43) | No | 25 (21) | 27 (23) | 16 (14) | 6 (5) | 2 (2) | NA |
| Lougaris, 2019 [42] | Italy | 184 | P | 72 (39) | No | NA | NA | NA | NA | NA | 132 (72) |
| Moschese, 2019 [66] | Italy | 103 | P | 39 (38) | No | 11 (11) | 19 (18) | 13 (13) | NA | NA | NA |
| Dominguez, 2012 [43] | Spain | 330 | P | 62 (19) | No | 21 (6) | 10 (3) | 12 (4) | NA | 14 (4) | NA |
| Edwards, 2004 [41] | US | 127 | P + A | 16 (13) | No | NA | NA | NA | NA | NA | NA |
| Plebani, 1987 [92] | Italy | 80 | P | 20 (25) | No | 16 (20) | NA | 5 (6) | NA | NA | NA |
| Živković, 2019 [70] | Croatia | 95 | P | NA | Yes | 55 (58) | 56 (59) | 15 (16) | NA | NA | NA |
| Jorgensen, 2013 [39] | Iceland | 32 | A | 15 (47) | Yes | 6 (9) | 12 (38) | 21 (66) | 2 (6) | 2 (6) | NA |
| Abolhassani, 2015 [84] | Iran | 57 | P | 32 (56) | No | 17 (30) | NA | NA | NA | NA | NA |
| Gualdi, 2015 [74] | Italy | 102 | P | NA | No | NA | NA | 59 (58%) | NA | NA | NA |
| Magen, 2017 [61] | Israel | 374 | P + A | NA | Yes | NA | NA | 16 (4%) | NA | NA | NA |
| Papadopoulou, 2005 [83] | Greece | 20 | P | 11 (55) | No | 17 (85) | NA | NA | NA | NA | 11 (55) |
| Wang, 2020 [93] | China | 43 | P + A | 6 (14) | No | 0 | 0 | 0 | 0 | 0 | NA |
| Delavari, 2020 [79] | Iran | 116 | P | 33 (28) | No | 11 (9) | 5 (4) | 6 (5) | 2 (2) | 6 (5) | NA |
| Jacob, 2008 [54] | Brazil | 126 | P + A | 61 (48) | No | NA | NA | NA | NA | 0 | NA |
| Burgio, 1980 [68] | Italy | 50 | P | 12 (24) | No | NA | NA | NA | NA | NA | NA |
| De Laat, 1991 [94] | Netherlands | 40 | P | 12 (30) | No | 8 (20) | 6 (15) | 5 (13%) | NA | NA | NA |
4.4. Etiopathogenesis of Allergy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weber-Mzell, D.; Kotanko, P.; Hauer, A.C.; Goriup, U.; Haas, J.; Lanner, N.; Erwa, W.; Ahmaida, I.A.; Haitchi-Petnehazy, S.; Stenzel, M.; et al. Gender, age and seasonal effects on IgA deficiency: A study of 7293 Caucasians. Eur. J. Clin. Investig. 2004, 34, 224–228. [Google Scholar] [CrossRef]
- Yel, L. Selective IgA deficiency. J. Clin. Immunol. 2010, 30, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Al-Herz, W.; Bousfiha, A.; Casanova, J.-L.; Chatila, T.; Conley, M.E.; Cunningham-Rundles, C.; Etzioni, A.; Holland, S.M.; Klein, C.; et al. Primary Immunodeficiency Diseases: An Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J. Clin. Immunol. 2015, 35, 696–726. [Google Scholar] [CrossRef]
- Aghamohammadi, A.; Abolhassani, H.; Biglari, M.; Abolmaali, S.; Moazzami, K.; Tabatabaeiyan, M.; Asgarian-Omran, H.; Parvaneh, N.; Mirahmadian, M.; Rezaei, N. Analysis of Switched Memory B Cells in Patients with IgA Deficiency. Int. Arch. Allergy Immunol. 2011, 156, 462–468. [Google Scholar] [CrossRef]
- Nechvatalova, J.; Pikulova, Z.; Stikarovska, D.; Pesak, S.; Vlkova, M.; Litzman, J. B-lymphocyte Subpopulations in Patients with Selective IgA Deficiency. J. Clin. Immunol. 2012, 32, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, C. Physiology of IgA and IgA Deficiency. J. Clin. Immunol. 2001, 21, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Latif, A.; Tabassomi, F.; Abolhassani, H.; Azizi, G.; Rezaei, N.; Aghamohammadi, A. Clinical phenotype classification for selective immunoglobulin A deficiency. Expert Rev. Clin. Immunol. 2015, 11, 1245–1254. [Google Scholar] [CrossRef]
- De Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Litinskiy, M.B.; Nardelli, B.; Hilbert, D.M.; He, B.; Schaffer, A.; Casali, P.; Cerutti, A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002, 3, 822–829. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, A.J.; Yilmaz, B.; Limenitakis, J.P.; Ganal-Vonarburg, S.C. IgA Function in Relation to the Intestinal Microbiota. Annu. Rev. Immunol. 2018, 36, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.W.; Ding, J.L. The Unexplored Roles of Human Serum IgA. DNA Cell Biol. 2014, 33, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.S.; Cripps, A.W.; Brown, S. Suppression of leucocyte chemokinesis and chemotaxis by human IgA. Clin. Exp. Immunol. 1980, 40, 388–395. [Google Scholar] [PubMed]
- ESID—European Society for Immunodeficiencies. Available online: https://esid.org/Education/Diagnostic-Criteria-PID (accessed on 6 December 2021).
- Swain, S.; Selmi, C.; Gershwin, M.E.; Teuber, S.S. The clinical implications of selective IgA deficiency. J. Transl. Autoimmun. 2019, 2, 100025. [Google Scholar] [CrossRef]
- Hammarström, L.; Vorechovsky, I.; Webster, D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID). Clin. Exp. Immunol. 2000, 120, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, Y.; Sanaei, R.; Yazdani, R.; Shekarabi, M.; Falak, R.; Mohammadi, J.; Abolhassani, H.; Aghamohammadi, A. The Heterogeneous Pathogenesis of Selective Immunoglobulin A Deficiency. Int. Arch. Allergy Immunol. 2019, 179, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Borte, S.; Pan-Hammarström, Q.; Liu, C.; Sack, U.; Borte, M.; Wagner, U.; Graf, D.; Hammarström, L. Interleukin-21 restores immunoglobulin production ex vivo in patients with common variable immunodeficiency and selective IgA deficiency. Blood 2009, 114, 4089–4098. [Google Scholar] [CrossRef]
- Castigli, E.; Wilson, S.A.; Scott, S.; Dedeoglu, F.; Xu, S.; Lam, K.-P.; Bram, R.J.; Jabara, H.; Geha, R.S. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 2005, 201, 35–39. [Google Scholar] [CrossRef]
- Ucher, A.J.; Ranjit, S.; Kadungure, T.; Linehan, E.K.; Khair, L.; Xie, E.; Limauro, J.; Rauch, K.S.; Schrader, C.E.; Stavnezer, J. Mismatch Repair Proteins and AID Activity Are Required for the Dominant Negative Function of C-Terminally Deleted AID in Class Switching. J. Immunol. 2014, 193, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Mackay, F.; Kalled, S.L. TNF ligands and receptors in autoimmunity: An update. Curr. Opin. Immunol. 2002, 14, 783–790. [Google Scholar] [CrossRef]
- Castigli, E.; Scott, S.; Dedeoglu, F.; Bryce, P.; Jabara, H.; Bhan, A.K.; Mizoguchi, E.; Geha, R.S. Impaired IgA class switching in APRIL-deficient mice. Proc. Natl. Acad. Sci. USA 2004, 101, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Castigli, E.; Wilson, S.A.; Garibyan, L.; Rachid, R.; Bonilla, F.; Schneider, L.; Geha, R.S. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat. Genet. 2005, 37, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Grosserichter-Wagener, C.; Franco-Gallego, A.; Ahmadi, F.; Moncada-Vélez, M.; Dalm, V.A.; Rojas, J.L.; Orrego, J.C.; Correa Vargas, N.; Hammarström, L.; Schreurs, M.W.; et al. Defective formation of IgA memory B cells, Th1 and Th17 cells in symptomatic patients with selective IgA deficiency. Clin. Transl. Immunol. 2020, 9, e1130. [Google Scholar] [CrossRef] [PubMed]
- Wing, J.B.; Lim, E.L.; Sakaguchi, S. Control of foreign Ag-specific Ab responses by Treg and Tfr. Immunol. Rev. 2020, 296, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Soheili, H.; Abolhassani, H.; Arandi, N.; Khazaei, H.A.; Shahinpour, S.; Hirbod-Mobarakeh, A.; Rezaei, N.; Aghamohammadi, A. Evaluation of Natural Regulatory T Cells in Subjects with Selective IgA Deficiency: From Senior Idea to Novel Opportunities. Int. Arch. Allergy Immunol. 2013, 160, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Azizi, G.; Abolhassani, H.; Aghamohammadi, A. Selective IgA Deficiency: Epidemiology, Pathogenesis, Clinical Phenotype, Diagnosis, Prognosis and Management. Scand. J. Immunol. 2017, 85, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Karaca, N.E.; Severcan, E.U.; Bilgin, B.G.; Azarsiz, E.; Akarcan, S.; Gunaydın, N.C.; Gulez, N.; Genel, F.; Aksu, G.; Kutukculer, N. Familial inheritance and screening of first-degree relatives in common variable immunodeficiency and immunoglobulin A deficiency patients. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418779458. [Google Scholar] [CrossRef]
- Pulvirenti, F.; Zuntini, R.; Milito, C.; Specchia, F.; Spadaro, G.; Danieli, M.G.; Pession, A.; Quinti, I.; Ferrari, S. Clinical Associations of Biallelic and Monoallelic TNFRSF13B Variants in Italian Primary Antibody Deficiency Syndromes. J. Immunol. Res. 2016, 2016, 8390356. [Google Scholar] [CrossRef]
- Aghamohammadi, A.; Mohammadi, J.; Parvaneh, N.; Rezaei, N.; Moin, M.; Espanol, T.; Hammarstrom, L. Progression of selective IgA deficiency to common variable immunodeficiency. Int. Arch. Allergy Immunol. 2008, 147, 87–92. [Google Scholar] [CrossRef]
- Vořechovský, I.; Webster, A.D.B.; Plebani, A.; Hammarström, L. Genetic Linkage of IgA Deficiency to the Major Histocompatibility Complex: Evidence for Allele Segregation Distortion, Parent-of-Origin Penetrance Differences, and the Role of Anti-IgA Antibodies in Disease Predisposition. Am. J. Hum. Genet. 1999, 64, 1096–1109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McBlane, J.F.; van Gent, D.C.; Ramsden, D.A.; Romeo, C.; Cuomo, C.A.; Gellert, M.; Oettinger, M.A. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell 1995, 83, 387–395. [Google Scholar] [CrossRef]
- Mohammadi, J.; Ramanujam, R.; Jarefors, S.; Rezaei, N.; Aghamohammadi, A.; Gregersen, P.K.; Hammarström, L. IgA deficiency and the MHC: Assessment of relative risk and microheterogeneity within the HLA A1 B8, DR3 (8.1) haplotype. J. Clin. Immunol. 2009, 30, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Chang, C.; Gershwin, M.E. IgA deficiency and autoimmunity. Autoimmun. Rev. 2014, 13, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Pan-Hammarström, Q.; Graham, R.R.; Gateva, V.; Fontán, G.; Lee, A.T.; Ortmann, W.; Urcelay, E.; Fernández-Arquero, M.; Núñez, C.; et al. Association of IFIH1 and other autoimmunity risk alleles with selective IgA deficiency. Nat. Genet. 2010, 42, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Karlsson, G.; Hansson, G.; Petruson, B.; Björkander, J.; Hanson, L.A. The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgM-producing cells in their nasal mucosa. Clin. Exp. Immunol. 1987, 67, 626–636. [Google Scholar] [PubMed]
- Morawska, I.; Kurkowska, S.; Bębnowska, D.; Hrynkiewicz, R.; Becht, R.; Michalski, A.; Piwowarska-Bilska, H.; Birkenfeld, B.; Załuska-Ogryzek, K.; Grywalska, E.; et al. The Epidemiology and Clinical Presentations of Atopic Diseases in Selective IgA Deficiency. J. Clin. Med. 2021, 10, 3809. [Google Scholar] [CrossRef]
- Jorgensen, G.H.; Gardulf, A.; Sigurdsson, M.I.; Sigurdardottir, S.T.; Thorsteinsdottir, I.; Gudmundsson, S.; Hammarström, L.; Ludviksson, B.R. Clinical symptoms in adults with selective IgA deficiency: A case-control study. J. Clin. Immunol. 2013, 33, 742–747. [Google Scholar] [CrossRef]
- Ocampo, C.J.; Peters, A.T. Antibody deficiency in chronic rhinosinusitis: Epidemiology and burden of illness. Am. J. Rhinol. Allergy 2013, 27, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.; Razvi, S.; Cunningham-Rundles, C. IgA deficiency: Clinical correlates and responses to pneumococcal vaccine. Clin. Immunol. 2004, 111, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Lougaris, V.; Sorlini, A.; Monfredini, C.; Ingrasciotta, G.; Caravaggio, A.; Lorenzini, T.; Baronio, M.; Cattalini, M.; Meini, A.; Ruggeri, L.; et al. Clinical and Laboratory Features of 184 Italian Pediatric Patients Affected with Selective IgA Deficiency (SIgAD): A Longitudinal Single-Center Study. J. Clin. Immunol. 2019, 39, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, O.; Giner, M.T.; Alsina, L.; Martín, M.A.; Lozano, J.; Plaza, A.M. Fenotipos clínicos asociados a la deficiencia selectiva de IgA revisión de 330 casos y propuesta de un protocolo de seguimiento. Anales Pediatria 2012, 76, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Chipps, B.E.; Talamo, R.C.; Winkelstein, J.A. IgA deficiency, recurrent pneumonias, and bronchiectasis. Chest 1978, 73, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, H.; Atlihan, F.; Genel, F.; Targan, S.; Gunvar, T. IgA and/or IgG subclass deficiency in children with recurrent respiratory infections and its relationship with chronic pulmonary damage. J. Investig. Allergol. Clin. Immunol. 2005, 15, 69–74. [Google Scholar] [PubMed]
- Hanson, L.A.; Söderström, R.; Nilssen, D.E.; Theman, K.; Björkander, J.; Söderström, T.; Karlsson, G.; Brandtzaeg, P. IgG subclass deficiency with or without IgA deficiency. Clin. Immunol. Immunopathol. 1991, 61, S70–S77. [Google Scholar] [CrossRef]
- French, M.A.; Denis, K.A.; Dawkins, R.; Peter, J.B. Severity of infections in IgA deficiency: Correlation with decreased serum antibodies to pneumococcal polysaccharides and decreased serum IgG2 and/or IgG4. Clin. Exp. Immunol. 1995, 100, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Costa Carvalho, B.T.; Nagao, A.T.; Arslanian, C.; Carneiro Sampaio, M.M.S.; Naspitz, C.K.; Sorensen, R.U.; Leiva, L.; Solé, D. Immunological evaluation of allergic respiratory children with recurrent sinusitis. Pediatr. Allergy Immunol. 2005, 16, 534–538. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Khan, D.A.; Ballas, Z.K.; Chinen, J.; Frank, M.M.; Hsu, J.T.; Keller, M.; Kobrynski, L.J.; Komarow, H.D.; Mazer, B.; et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J. Allergy Clin. Immunol. 2015, 136, 1186–1205.e78. [Google Scholar] [CrossRef] [PubMed]
- Zinneman, H.H.; Kaplan, A.P. The association of giardiasis with reduced intestinal secretory immunoglobulin A. Dig. Dis. Sci. 1972, 17, 793–797. [Google Scholar] [CrossRef]
- Meini, A.; Pillan, N.M.; Villanacci, V.; Monafo, V.; Ugazio, A.G.; Plebani, A. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann. Allergy Asthma Immunol. 1996, 77, 333–336. [Google Scholar] [CrossRef]
- Cunningham-Rundles, C.; Brandeis, W.E.; Pudifin, D.J.; Day, N.K.; Good, R.A. Autoimmunity in selective IgA deficiency: Relationship to anti-bovine protein antibodies, circulating immune complexes and clinical disease. Clin. Exp. Immunol. 1981, 45, 299–304. [Google Scholar] [PubMed]
- Odineal, D.D.; Gershwin, M.E. The Epidemiology and Clinical Manifestations of Autoimmunity in Selective IgA Deficiency. Clin. Rev. Allergy Immunol. 2020, 58, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.M.A.; Pastorino, A.C.; Fahl, K.; Carneiro-Sampaio, M.; Monteiro, R.C. Autoimmunity in IgA deficiency: Revisiting the role of IgA as a silent housekeeper. J. Clin. Immunol. 2008, 28, S56–S61. [Google Scholar] [CrossRef] [PubMed]
- Sandler, S.G.; Mallory, D.; Malamut, D.; Eckrich, R. IgA anaphylactic transfusion reactions. Transfus. Med. Rev. 1995, 9, 1–8. [Google Scholar] [CrossRef]
- Quiding-Järbrink, M.; Sundström, P.; Lundgren, A.; Hansson, M.; Bäckström, M.; Johansson, C.; Enarsson, K.; Hermansson, M.; Johnsson, E.; Svennerholm, A.M. Decreased IgA antibody production in the stomach of gastric adenocarcinoma patients. Clin. Immunol. 2009, 131, 463–471. [Google Scholar] [CrossRef]
- Kersey, J.H.; Shapiro, R.S.; Filipovich, A.H. Relationship of immunodeficiency to lymphoid malignancy. Pediatr. Infect. Dis. J. 1988, 7 (Suppl. S5), S10–S12. [Google Scholar] [CrossRef]
- Mellemkjaer, L.; Hammarstrom, L.; Andersen, V.; Yuen, J.; Heilmann, C.; Barington, T.; Bjorkander, J.; Olsen, J.H. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: A combined Danish and Swedish study. Clin. Exp. Immunol. 2002, 130, 495–500. [Google Scholar] [CrossRef]
- Zhang, J.; van Oostrom, D.; Li, J.; Savelkoul, H.F.J. Innate Mechanisms in Selective IgA Deficiency. Front. Immunol. 2021, 12, 649112. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Agache, I.; Bavbek, S.; Bilo, B.M.; Braido, F.; Cardona, V.; Custovic, A.; Demonchy, J.; Demoly, P.; Eigenmann, P.; et al. Research needs in allergy: An EAACI position paper in collaboration with, E.F.A. Clin. Transl. Allergy 2012, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Magen, E.; Masalha, A.; Waitman, D.A.; Kahan, N.; Viner, I.; Klassov, L.; Vardy, D. Prevalence of dermatologic diseases among patients with selective immunoglobulin A deficiency. Allergy Asthma Proc. 2017, 38, 70–77. [Google Scholar] [CrossRef]
- Janzi, M.; Kull, I.; Sjöberg, R.; Wan, J.; Melén, E.; Bayat, N.; Ostblom, E.; Pan-Hammarström, Q.; Nilsson, P.; Hammarström, L. Selective IgA deficiency in early life: Association to infections and allergic diseases during childhood. Clin. Immunol. 2009, 133, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Buckley, R.H.; Dees, S.C. Correlation of milk precipitins with IgA deficiency. New Engl. J. Med. 1969, 281, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Klemola, T. Deficiency of immunoglobulin A. Ann. Clin. Res. 1987, 19, 248–257. [Google Scholar] [PubMed]
- Aytekin, C.; Tuygun, N.; Gokce, S.; Dogu, F.; Ikinciogullari, A. Selective IgA deficiency: Clinical and laboratory features of 118 children in Turkey. J. Clin. Immunol. 2012, 32, 961–966. [Google Scholar] [CrossRef]
- Moschese, V.; Chini, L.; Graziani, S.; Sgrulletti, M.; Gallo, V.; Di Matteo, G.; Ferrari, S.; Di Cesare, S.; Cirillo, E.; Pession, A.; et al. Follow-up and outcome of symptomatic partial or absolute IgA deficiency in children. Eur. J. Nucl. Med. Mol. Imaging 2018, 178, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, A.; Cheraghi, T.; Gharagozlou, M.; Movahedi, M.; Rezaei, N.; Yeganeh, M.; Parvaneh, N.; Abolhassani, H.; Pourpak, Z.; Moin, M. IgA deficiency: Correlation between clinical and immunological phenotypes. J. Clin. Immunol. 2009, 29, 130–136. [Google Scholar] [CrossRef]
- Burgio, G.R.; Duse, M.; Monafo, V.; Ascione, A.; Nespoli, L. Selective IgA deficiency: Clinical and immunological evaluation of 50 pediatric patients. Eur J Pediatr. 1980, 133, 101–106. [Google Scholar] [CrossRef]
- Erkoçoğlu, M.; Metin, A.; Kaya, A.; Özcan, C.; Akan, A.; Civelek, E.; Çapanoğlu, M.; Giniş, T.; Kocabaş, C.N. Allergic and autoimmune disorders in families with selective IgA deficiency. Turk. J. Med Sci. 2017, 47, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Živković, J.; Lipej, M.; Banić, I.; Bulat Lokas, S.; Nogalo, B.; Lulić Jurjević, R.; Turkalj, M. Respiratory and allergic disorders in children with severe and partial immunoglobulin A immunodeficiency. Scand. J. Immunol. 2019, 90, e12828. [Google Scholar] [CrossRef] [PubMed]
- Scala, E.; Villalta, D.; Uasuf, C.G.; Pignatti, P.; Pirrotta, L.; Guerra, E.C.; Locanto, M.; Meneguzzi, G.; Giani, M.; Cecchi, L.; et al. An atlas of IgE sensitization patterns in different Italian areas. A multicenter, cross-sectional study. Eur. Ann. Allergy Clin. Immunol. 2018, 50, 217–225. [Google Scholar] [CrossRef]
- Galli, E.; Cinicola, B.; Carello, R.; Caimmi, S.; Brindisi, G.; De Castro, G.; Manti, S.; Martelli, A. Atopic dermatitis. Acta Biomed. 2020, 91, e2020011. [Google Scholar] [PubMed]
- Dizon, M.P.; Yu, A.M.; Singh, R.K.; Wan, J.; Chren, M.-M.; Flohr, C.; Silverberg, J.I.; Margolis, D.J.; Langan, S.M.; Abuabara, K. Systematic review of atopic dermatitis disease definition in studies using routinely collected health data. Br. J. Dermatol. 2018, 178, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Lougaris, V.; Baronio, M.; Vitali, M.; Tampella, G.; Moratto, D.; Tanghetti, P.; Monari, P.; Calzavara-Pinton, P.; Plebani, A. Burden of Skin Disease in Selective IgA Deficiency and Common Variable Immunodeficiency. J. Investig. Allergol Clin. Immunol. 2015, 25, 369–371. [Google Scholar] [PubMed]
- Latcham, F.; Merino, F.; Lang, A.; Garvey, J.; Thomson, M.A.; Walker-Smith, J.A.; Davies, S.E.; Phillips, A.D.; Murch, S.H. A consistent pattern of minor immunodeficiency and subtle enteropathy in children with multiple food allergy. J. Pediatr. 2003, 143, 39–47. [Google Scholar] [CrossRef]
- Shahin, R.Y.; Ali, F.H.A.; Melek, N.A.N.; Elateef, I.A.A.; Attia, M.Y. Study of selective immunoglobulin A deficiency among Egyptian patients with food allergy. Central Eur. J. Immunol. 2020, 45, 184–188. [Google Scholar] [CrossRef]
- Tuano, K.S.; Orange, J.S.; Sullivan, K.; Cunningham-Rundles, C.; Bonilla, F.A.; Davis, C.M. Food allergy in patients with primary immunodeficiency diseases: Prevalence within the US Immunodeficiency Network (USIDNET). J. Allergy Clin. Immunol. 2015, 135, 273–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Heal. 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Delavari, S.; Shad, T.M.; Shariati, S.; Salami, F.; Rasouli, S. Allergy in Patients with Selective IgA Deficiency. Immunol. Genet. J. 2020, 10, 54–63. [Google Scholar]
- Kim, W.-J.; Choi, I.S.; Kim, C.S.; Lee, J.-H.; Kang, H.-W. Relationship between serum IgA level and allergy/asthma. Korean J. Intern. Med. 2017, 32, 137–145. [Google Scholar] [CrossRef]
- Lúdvíksson, B.R.; Eiríksson, T.H.; Ardal, B.; Sigfússon, A.; Valdimarsson, H. Correlation between serum immunoglobulin A concentrations and allergic manifestations in infants. J. Pediatr. 1992, 121, 23–27. [Google Scholar] [CrossRef]
- Sandin, A.; Björkstén, B.; Böttcher, M.F.; Englund, E.; Jenmalm, M.C.; Bråbäck, L. High salivary secretory IgA antibody levels are associated with less late-onset wheezing in IgE-sensitized infants. Pediatr. Allergy Immunol. 2011, 22, 477–481. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Mermiri, D.; Taousani, S.; Triga, M.; Nicolaidou, P.; Priftis, K.N. Bronchial hyper-responsiveness in selective IgA deficiency. Pediatr. Allergy Immunol. 2005, 16, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, H.; Gharib, B.; Shahinpour, S.; Masoom, S.N.; Havaei, A.; Mirminachi, B.; Arandi, N.; Torabi-Sagvand, B.; Khazaei, H.A.; Mohammadi, J.; et al. Autoimmunity in patients with selective IgA deficiency. J. Investig. Allergol. Clin. Immunol. 2015, 25, 112–119. [Google Scholar] [PubMed]
- Urm, S.-H.; Yun, H.D.; Fenta, Y.A.; Yoo, K.H.; Abraham, R.S.; Hagan, J.; Juhn, Y.J. Asthma and risk of selective IgA deficiency or common variable immunodeficiency: A population-based case-control study. Mayo Clin. Proc. 2013, 88, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Geng, B.; Cameron, D.W.; Murphy, L.M.; Schulman, E.S. Primary immune deficiency diseases as unrecognized causes of chronic respiratory disease. Respir. Med. 2017, 132, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Reisi, M.; Azizi, G.; Kiaee, F.; Masiha, F.; Shirzadi, R.; Momen, T.; Rafiemanesh, H.; Tavakolinia, N.; Modaresi, M.; Aghamohammadi, A. Evaluation of pulmonary complications in patients with primary immunodeficiency disorders. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 122–128. [Google Scholar] [PubMed]
- Custovic, A.; Murray, C.; Simpson, A. Allergy and infection: Understanding their relationship. Allergy 2005, 60 (Suppl. S79), 10–13. [Google Scholar] [CrossRef]
- Juhn, Y.J. Risks for infection in patients with asthma (or other atopic conditions): Is asthma more than a chronic airway disease? J. Allergy Clin. Immunol. 2014, 134, 247–257. [Google Scholar] [CrossRef]
- De Moraes Lui, C.; Oliveira, L.C.; Diogo, C.L.; Kirschfink, M.; Grumach, A.S. Immunoglobulin G subclass concentrations and infections in children and adolescents with severe asthma. Pediatr. Allergy Immunol. 2002, 13, 195–202. [Google Scholar] [CrossRef]
- Shkalim, V.; Monselize, Y.; Segal, N.; Zan-Bar, I.; Hoffer, V.; Garty, B.Z. Selective IgA deficiency in children in Israel. J. Clin. Immunol. 2010, 30, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Plebani, A.; Monafo, V.; Ugazio, A.G.; Monti, C.; Avanzini, M.A.; Massimi, P.; Burgio, G.R. Comparison of the frequency of atopic diseases in children with severe and partial IgA deficiency. Int. Arch. Allergy Immunol. 1987, 82, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yao, T.; Zhang, T.; Quan, M.; Wang, C.; Wang, C.; Zhang, L.; Tang, X.; Jian, S.; Song, H. Selective immunoglobulin A deficiency (SIgAD) primarily leads to recurrent infections and autoimmune diseases: A retrospective study of Chinese patients in the past 40 years. Genes Dis. 2020, 7, 115–121. [Google Scholar] [CrossRef] [PubMed]
- De Laat, P.C.; Weemaes, C.M.; Gonera, R.; Van Munster, P.J.; Bakkeren, J.A.; Stoelinga, G.B. Clinical manifestations in selective IgA deficiency in childhood. A follow-up report. Acta Paediatr. 1991, 80, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 2012, 12, 821–832. [Google Scholar] [CrossRef]
- Latiff, A.H.A.; Kerr, M.A. The clinical significance of immunoglobulin A deficiency. Ann. Clin. Biochem. Int. J. Lab. Med. 2007, 44, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.R.; Johansen, F.-E.; Kahu, H.; Macpherson, A.; Brandtzaeg, P. Hypersensitivity and oral tolerance in the absence of a secretory immune system. Allergy 2010, 65, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Cripps, A.W.; Clancy, R.L.; Hensley, M.J.; Henry, R.J.; Wlodarczyk, J.H. The significance of transient mucosal IgA deficiency on the development of asthma and atopy in children. Adv. Exp. Med. Biol. 1995, 371B, 861–864. [Google Scholar] [PubMed]
- Sloper, K.S.; Brook, C.G.; Kingston, D.; Pearson, J.R.; Shiner, M. Eczema and atopy in early childhood: Low IgA plasma cell counts in the jejunal mucosa. Arch. Dis. Child. 1981, 56, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.M.; Laine, S.T.; Järvenpää, A.L.; Suomalainen, H.K. Does low IgA in human milk predispose the infant to development of cow’s milk allergy? Pediatr. Res. 2000, 48, 457–462. [Google Scholar] [CrossRef][Green Version]
- Breedveld, A.; van Egmond, M. IgA and FcαRI: Pathological Roles and Therapeutic Opportunities. Front. Immunol. 2019, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.S.; Larché, M.; Durham, S.R. Tregs and allergic disease. J. Clin. Invest. 2004, 114, 1389–1397. [Google Scholar] [CrossRef]
- Pilette, C.; Ouadrhiri, Y.; Godding, V.; Vaerman, J.P.; Sibille, Y. Lung mucosal immunity: Immunoglobulin-A revisited. Eur. Respir. J. 2001, 18, 571–588. [Google Scholar] [CrossRef]
- Strait, R.T.; Mahler, A.; Hogan, S.; Khodoun, M.; Shibuya, A.; Finkelman, F.D. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J. Allergy Clin. Immunol. 2011, 127, 982–989.e1. [Google Scholar] [CrossRef] [PubMed]
- Szczawińska-Popłonyk, A.; Bręborowicz, A.; Ossowska, L. Food Allergy in Children with Hypogammaglobulinemia. Pediatr. Pol. 2012, 87, 444–448. Available online: https://cyberleninka.org/article/n/498458 (accessed on 6 December 2021). [CrossRef]
- Abokor, A.A.; McDaniel, G.H.; Golonka, R.M.; Campbell, C.; Brahmandam, S.; Yeoh, B.S.; Joe, B.; Vijay-Kumar, M.; Saha, P. Immunoglobulin A, an Active Liaison for Host-Microbiota Homeostasis. Microorganisms 2021, 9, 2117. [Google Scholar] [CrossRef]
- Fadlallah, J.; El Kafsi, H.; Sterlin, D.; Juste, C.; Parizot, C.; Dorgham, K.; Autaa, G.; Gouas, D.; Almeida, M.; Lepage, P.; et al. Microbial ecology perturbation in human IgA deficiency. Sci. Transl. Med. 2018, 10, eaan1217. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Fadlallah, J.; Adams, O.; Fieschi, C.; Parizot, C.; Dorgham, K.; Rajkumar, A.; Autaa, G.; El-Kafsi, H.; Charuel, J.L.; et al. Human IgA binds a diverse array of commensal bacteria. J. Exp. Med. 2020, 217, e20181635. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, J.R.; Strauss, J.D.; Bielecka, A.; Porto, A.F.; Lobo, F.M.; Urban, A.; Schofield, W.B.; Palm, N.W. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory, I.g.M. Sci. Rep. 2019, 9, 13574. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, J.; Sterlin, D.; Fieschi, C.; Parizot, C.; Dorgham, K.; El Kafsi, H.; Autaa, G.; Ghillani-Dalbin, P.; Juste, C.; Lepage, P.; et al. Synergistic convergence of microbiota-specific systemic, I.g.G.; secretory, I.g.A. J. Allergy Clin. Immunol. 2019, 143, 1575–1585.e4. [Google Scholar] [CrossRef] [PubMed]
- Moll, J.M.; Myers, P.N.; Zhang, C.; Eriksen, C.; Wolf, J.; Appelberg, K.S.; Lindberg, G.; Bahl, M.I.; Zhao, H.; Pan-Hammarström, Q.; et al. Gut Microbiota Perturbation in IgA Deficiency Is Influenced by IgA-Autoantibody Status. Gastroenterology 2021, 160, 2423–2434.e5. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz Peña, M.J.; Gonzalez-Granado, L.I.; Garcia-Heredia, I.; Carballa, L.M.; Martinez-Garcia, M. Minimal-moderate variation of human oral virome and microbiome in IgA deficiency. Sci. Rep. 2021, 11, 14913. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Antimalarial Agents |
| Carbamazepine | |
| Valproate | |
| Glucocorticoids | |
| Fenclofenac | |
| Gold salts | |
| Penicillamine | |
| Sulfasalazine | |
| Infections | Congenital Rubella |
| Congenital Cytomegalovirus Infection | |
| Congenital Toxoplasma Gondii Infection | |
| Epstein-Barr Virus | |
| Monogenic disease | Ataxia-telangiectasia |
| Wiskott-Aldrich Syndrome | |
| X-linked lymphoproliferative disease | |
| Transcobalamin II deficiency | |
| Chromosomal abnormalities | Monosomy 22 |
| Deletion syndrome of chromosome 18q | |
| Trisomy 22 | |
| Trisomy 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinicola, B.L.; Pulvirenti, F.; Capponi, M.; Bonetti, M.; Brindisi, G.; Gori, A.; De Castro, G.; Anania, C.; Duse, M.; Zicari, A.M. Selective IgA Deficiency and Allergy: A Fresh Look to an Old Story. Medicina 2022, 58, 129. https://doi.org/10.3390/medicina58010129
Cinicola BL, Pulvirenti F, Capponi M, Bonetti M, Brindisi G, Gori A, De Castro G, Anania C, Duse M, Zicari AM. Selective IgA Deficiency and Allergy: A Fresh Look to an Old Story. Medicina. 2022; 58(1):129. https://doi.org/10.3390/medicina58010129
Chicago/Turabian StyleCinicola, Bianca Laura, Federica Pulvirenti, Martina Capponi, Marta Bonetti, Giulia Brindisi, Alessandra Gori, Giovanna De Castro, Caterina Anania, Marzia Duse, and Anna Maria Zicari. 2022. "Selective IgA Deficiency and Allergy: A Fresh Look to an Old Story" Medicina 58, no. 1: 129. https://doi.org/10.3390/medicina58010129
APA StyleCinicola, B. L., Pulvirenti, F., Capponi, M., Bonetti, M., Brindisi, G., Gori, A., De Castro, G., Anania, C., Duse, M., & Zicari, A. M. (2022). Selective IgA Deficiency and Allergy: A Fresh Look to an Old Story. Medicina, 58(1), 129. https://doi.org/10.3390/medicina58010129






