Abstract
Background and objectives: Current guidelines criteria do not satisfactorily discriminate responders to cardiac resynchronization therapy (CRT). QRS amplitude is an established index to recognize the severity of myocardial disturbance and might be a key to optimal patient selection for CRT. Materials and Methods: (1) Initial R-wave amplitude, (2) S-wave amplitude, and (3) a summation of maximal R- or R′-wave amplitude and S-wave amplitude were measured at baseline. These parameters were averaged according to right (V1 to V3) or left (V4 to V6) precordial leads. The impact of these parameters on response to CRT, which was defined as a decrease in left ventricular end-systolic volume ≥15% at six-month follow-up, was investigated. Results: Among 47 patients (71 years old, 28 men) who received guideline-indicated CRT implantation, 25 (53%) achieved the definition of CRT responder. Among baseline electrocardiogram parameters, only the higher S-wave amplitude in right precordial leads was an independent predictor of CRT responders (odds ratio: 2.181, 95% confidence interval: 1.078–4.414, p = 0.030) at a cutoff of 1.44 mV. The cutoff was independently associated with cumulative incidence of heart failure readmission and appropriate electrical defibrillation following CRT implantation (p < 0.05, respectively). Conclusions: Prominent S-wave in right precordial leads might be a promising index to predict left ventricular reverse remodeling and greater clinical outcomes following CRT implantation.
1. Introduction
Guidelines [1,2,3] state that cardiac resynchronization therapy (CRT) improves clinical outcomes in strictly selected heart failure patients, particularly those with left bundle branch block (LBBB). Previous studies suggested several baseline characteristics, including PR interval, QRS duration, and QRS axis in surface electrocardiogram, as additional predictors of favorable responses to CRT, accompanying cardiac reverse remodeling and greater clinical outcomes [4,5,6,7]. Of note, a multicenter study recently demonstrated that a decrease in QRS amplitude at lead V1 after CRT implantation could predict favorable outcomes [8]. Nevertheless, various indices, including guidelines criteria, cannot satisfactory discriminate responders to CRT, and further studies are warranted to propose an optimal index to predict responders to CRT.
QRS amplitude is an established index to recognize myocardial damage or replacement of fibrosis in various cardiac disorders [9,10,11,12]. Echocardiographic response and favorable outcomes following CRT implantation would be affected by the myocardial viability [13,14,15]. Therefore, we hypothesized that baseline QRS amplitude in precordial leads might be a novel predictor of CRT responders in addition to the current guidelines criteria. We aimed to assess the implication of baseline QRS amplitude in precordial leads in predicting CRT responders.
2. Methods
2.1. Study Population
A total of 69 heart failure patients who received CRT implantation between March 2010 and December 2020 at our institute were included in this retrospective study. All patients met the following guideline-directed criteria: (1) New York Heart Association functional class II–IV; (2) left ventricular ejection fraction ≤35% or <50% if dependent on right ventricular pacing rhythm; (3) QRS duration ≥120 ms. The study was conducted in compliance with the Declaration of Helsinki and was approved by the institutional review board at the University of Toyama. Informed consent was obtained from all patients.
2.2. Clinical Characteristics
Baseline clinical characteristics such as demographic, laboratory, and echocardiographic parameters were retrieved from the electronic medical records.
2.3. Standard 12-Lead Electrocardiograms
Electrocardiograms (ECGs) were recorded with an amplification of 1 cm/mV. Parameters such as QRS axis, QRS duration in lead II, QRS morphology classified into right bundle branch block, LBBB, and intraventricular conduction disturbance were measured. In precordial leads, initial R-wave amplitude, S-wave amplitude, and QRS amplitude consisting of maximal R- or R′-wave amplitude plus S-wave amplitude were measured and were averaged according to right (V1 to V3) or left (V4 to V6) precordial leads (see Supplementary Figure S1 as an example). These parameters were averaged among three consecutive beats in cases of atrial fibrillation. In cases of right ventricular pacing dependency, baseline ECGs were defined as the parameters during right ventricular pacing. ECGs were reviewed independently by three investigators in a blinded manner (NK, TK, and KU). If there was considerable discrepancy, consensus was obtained following detailed discussion among them.
2.4. Echocardiograms and CRT Responder (Primary Endpoint)
Variables obtained by an echocardiogram (left atrial dimension, left ventricular end-diastolic/-systolic dimension, left ventricular end-systolic volume (LVESV), and left ventricular ejection fraction (LVEF)) were collected within one week before CRT implantation (baseline) and six months later. A CRT responder was defined as a subject who achieved a reduction of LVESV ≥ 15% at six months following CRT implantation as a primary endpoint [16]. Patients who received a heart transplant or a left ventricular assist device prior to the end of follow-up period were assigned to the non-responders.
2.5. Clinical Outcomes (Secondary Endpoint)
Clinical events including a composite endpoint (cardiovascular death, a heart transplantation, or a left ventricular assist device implantation), worsening of heart failure requiring unplanned hospitalization, and appropriate electrical defibrillation for ventricular tachyarrhythmias (only for those with implanted defibrillators) were counted.
2.6. Statistical Analysis
Two-sided p-value < 0.05 was considered as statistically significant. Data analysis was performed using SPSS v16.0 (IBM, New York, NY, USA). Data were expressed as the mean and standard deviation for normally distributed variables and as the median with the interquartile range for non-normally distributed data. Continuous data were compared using t-test or the Mann–Whitney test, as appropriate. Categorical data were expressed as numbers and percentages and compared using chi-squared test.
Logistic regression analyses were performed to investigate the predictors of CRT responders among baseline variables, including ECG parameters. Multivariate analyses were performed for those with p < 0.05 in the univariate analyses. Receiver-operating characteristics analysis was performed to calculate a cutoff of continuous variables to predict CRT responders.
Cumulative incidence of clinical events was stratified by the cutoff of independent variables and compared between the two groups using a log-rank test. Univariate and multivariate Cox proportional hazard ratio regression analyses were performed to investigate the impact of ECG parameters on clinical outcomes, which were adjusted for other considerable variables, including age, male sex, ischemic etiology, left ventricular ejection fraction, the serum levels of B-type natriuretic peptide, and LBBB [2,17,18].
3. Results
3.1. Patient Characteristics
Among 69 patients included in this study, 22 without follow-up echocardiograms were excluded from the primary analyses and only included in the secondary analyses.
A total of 47 patients who received CRT implantation and completed paired echocardiograms tests were included in the primary analyses (Table 1). Twenty-five (53%) patients were assigned to the responders. There were no significant differences in baseline characteristics except for the higher prevalence of beta-blocker use in the responders. As for the electrocardiogram data, QRS morphology was not different between the two, and QRS axis deviated to the right in the responders. Averaged QRS amplitude in right precordial leads, averaged S-wave amplitude in right precordial leads, and averaged S-wave amplitude in left precordial leads were significantly higher in the responders.

Table 1.
Comparison of clinical characteristics between responder and non-responder.
3.2. Impact of QRS Amplitude on CRT Response
Averaged QRS amplitude in right precordial leads, averaged S-wave amplitude in right precordial leads, and averaged S-wave amplitude in left precordial leads were analyzed by multivariate logistic regression analysis adjusted for beta-blockers, systolic blood pressure, and QRS axis, which were significant in the univariate analyses (Table 2). Finally, only the averaged S-wave amplitude in right precordial leads was significantly associated with CRT responder.

Table 2.
Logistic regression analyses of echocardiographic responder.
ROC analysis showed a cutoff of 1.44 mV for the S-wave amplitude in right precordial leads to best predict CRT responder, with an area under the curve of 0.787, sensitivity of 84.0%, and specificity of 68.2% (Figure 1). Representative ECGs of responder and non-responder are displayed in Figure 2A–D. Especially in Figure 2C,D (patients with right bundle branch block), notable S-waves were observed in responder (Figure 2C); whereas there were few S-waves in non-responder (Figure 2D).
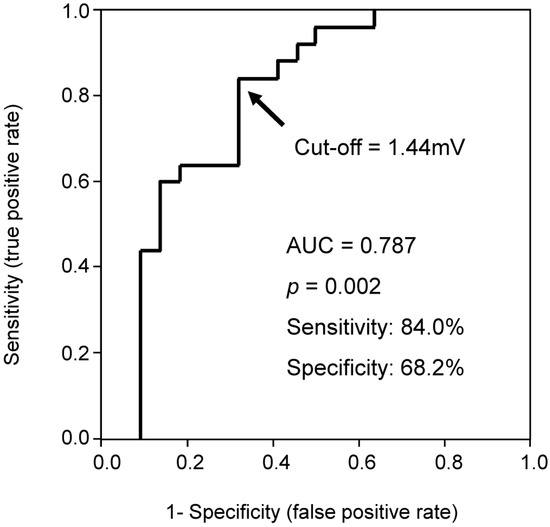
Figure 1.
Receiver-operating characteristic curve of averaged S-wave amplitude in right precordial leads for predicting CRT (cardiac resynchronization therapy) responder.
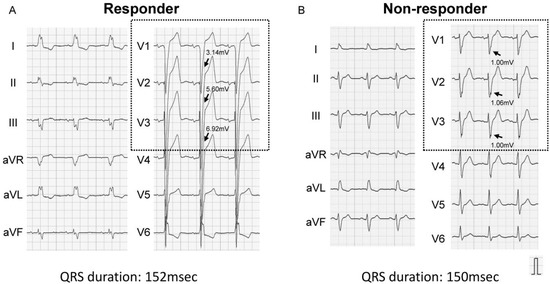
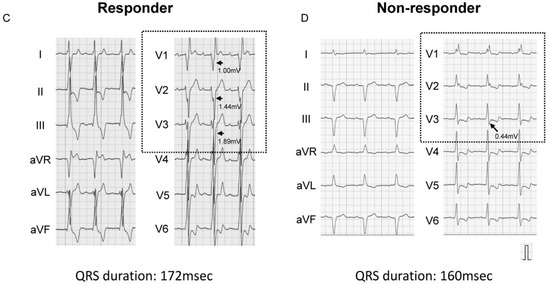
Figure 2.
Representative baseline electrocardiograms (left bundle branch block (A,B), right bundle branch block (C,D)). Arrows indicate S-waves in right precordial leads. (A) Left bundle branch block and prominent S-wave: CRT responder; (B) Atypical left bundle branch block and small S-wave: CRT non-responder; (C) Atypical right bundle branch block and notable S-wave: CRT responder; (D) Right bundle branch block and few S-waves: CRT non-responder.
Among 69 patients who had at least baseline ECGs, baseline characteristics between patients with averaged S-wave in right precordial leads < 1.44 mV and those with S-wave ≥ 1.44 mV were compared (Table 3). Patients with S-wave ≥ 1.44 mV represented significantly lower left atrial dimension, higher incidence of LBBB, and more left axis deviation compared with patients with S-wave < 1.44 mV. The previous episodes of ventricular tachyarrhythmias including sustained ventricular tachycardia or ventricular fibrillation are not significantly different between the two groups.

Table 3.
Comparison of baseline characteristics between patients with averaged S-wave in right precordial leads <1.44 mV and those with averaged S-wave ≥ 1.44 mV.
3.3. Impact of S-Wave Amplitude in Right Precordial Leads on Clinical Outcomes
Among 69 patients (median 515 (261–1583) days follow-up) who had at least baseline ECGs, cumulative incidences of the composite endpoint, heart failure readmission, and appropriate electrical defibrillation (applicable for 53 patients with implanted defibrillators) were lower in the patients with higher S-wave amplitude in V1–3 (Figure 3A–C). Cox proportional hazard ratio regression analyses of averaged S-wave amplitude in right precordial leads were performed for predicting clinical outcomes (Table 4). The high averaged S-wave in right precordial leads was associated with the freedom from heart failure readmission and appropriate electrical defibrillation (p < 0.05 for both).
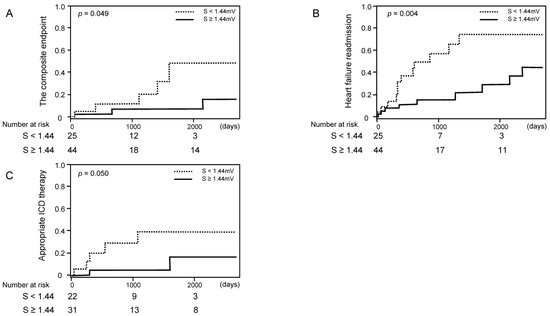
Figure 3.
Cumulative incidence of clinical events stratified by the cutoff of S-wave amplitude ((A) cardiovascular death; (B) heart failure readmission; (C) appropriate ICD (implantable cardioverter defibrillator) therapy).

Table 4.
Multivariate regression analyses for clinical outcomes.
When we excluded those with LBBB, similar trends remained, although some of them did not reach statistical significance except for heart failure readmission (Figure 4A–C).
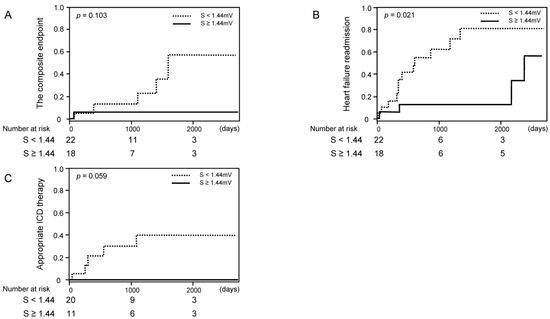
Figure 4.
Cumulative incidence of clinical events stratified by the cutoff of S-wave amplitude among those without left bundle branch block ((A) the composite endpoint of cardiovascular death, a heart transplantation, or a left ventricular assist device implantation; (B) heart failure readmission; (C) appropriate ICD therapy).
4. Discussion
We investigated the association between baseline QRS amplitude in precordial leads and echocardiographic response to CRT. Although we implanted CRT according to the guideline-recommended criteria, only 53% were CRT responders. The higher S-wave amplitude in right precordial leads was associated with greater cardiac reverse remodeling and clinical outcomes following CRT implantation.
4.1. CRT Responders
CRT improves left ventricular electrophysiological desynchrony using additional left ventricular lead, particularly in those with LBBB. However, not all candidates enjoy satisfactory cardiac reverse remodeling following guideline-indicated CRT implantation. Many recent studies define CRT responder as achieving a reduction of LVESV ≥15% in echocardiographic assessment following a certain period after CRT implantation [19]. Wide QRS duration and LBBB are well-known predictors of CRT responders, whereas recent studies have argued against the implication of QRS duration [20,21]. Given that the CRT non-responders have poor prognosis, optimal patient selection using appropriate predictors of CRT response is warranted in addition to the current guidelines criteria.
4.2. Implication of S-Wave Amplitude in Right Precordial Leads
The results of this study are supported by the fact that prominent S-waves in right precordial leads are observed in a typical LBBB. However, in this study, some patients were CRT responders and had good clinical outcomes irrespective of the existence of LBBB. Other studies consistently demonstrated that some patients with right bundle branch block or nonspecific ventricular conduction delay also showed favorable response to CRT despite the lack of LBBB [4,22]. Interestingly, prominent S-wave in right precordial leads was associated with lower incidence of electrical defibrillation following CRT implantation. CRT response leading to left ventricular reverse remodeling might prevent ventricular tachyarrhythmia.
We speculate that the presence of S-wave in right precordial leads might indicate conduction disturbance in the left ventricle irrespective of the type of bundle branch block. The existence of prominent S-wave, a novel and simple index to predict CRT responder that we propose here, might include most of LBBB and some part of non-LBBB.
Moreover, the existence of prominent S-wave was associated with small left atrium in this study. Previous studies described that the left atrial area and function are associated with CRT response [23,24]. The reasons why S-wave amplitude was associated with left atrial diameter are unclear; however, S-wave amplitude might represent a less remodeled left atrium with remaining responsibility to CRT.
4.3. Optimal Patient Selection for the Favorable Responses to CRT
The left axis deviation, which reflects a left anterior fascicular block, is an independent predictor of clinical outcomes following CRT implantation [7,25,26]. Alternatively, the left axis deviation appears not only in the conduction disturbance but also in the left ventricular hypertrophy and the myocardial infarction of the inferior wall. However, S-wave in right precordial leads is affected mainly by the activation of the left ventricle in a vector away from the right precordial leads [8]. We speculated that these differences might create a discrepancy in the predictive power between the left axis deviation and the prominent S-wave in right precordial leads in this study.
By adding the prominent S-wave in right precordial leads to the current guidelines criteria, we might be able to further discriminate CRT responders who can enjoy greater reverse remodeling and clinical outcomes, especially in a non-LBBB pattern such as in intraventricular conduction disturbance. Of note, given a high sensitivity, a prominent S-wave would be more useful to predict non-responders to CRT.
4.4. Study Limitations
First, this was a retrospective observational study including small sample size from a single center, and further prospective randomized larger and multicenter trials are needed. Second, the number of patients with ischemic heart disease was small compared with the previous large cohort, indicating selection bias might exist in this study. Of course, QRS morphology such as right bundle branch block or LBBB, which was not included in adjusted parameters for CRT response, should affect the S-wave amplitude impact. Third, although we used the use of beta-blocker for the adjustment, we cannot completely exclude the impact of beta-blocker use. Fourth, several factors such as pericardial effusion, obesity, and pulmonary emphysema might affect QRS amplitude. Finally, the development of new devices and techniques for the implantation might affect CRT response.
5. Conclusions
The existence of prominent S-wave in right precordial leads would be a key to further discriminate CRT responders and non-responders in addition to the current guideline criteria.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/medicina57080815/s1, Figure S1: Measurements of ECG parameters in precordial leads.
Author Contributions
Conceptualization, N.K. and T.I.; methodology, N.K.; software, T.I.; validation, N.K., T.I., T.K. and K.U.; formal analysis, T.I.; investigation, N.K.; resources, N.K.; data curation, T.K. and K.U.; writing—original draft preparation, N.K.; writing—review and editing, T.I. and K.K.; visualization, T.I.; supervision, K.K.; project administration, K.K.; funding acquisition, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by JSPS KAKENHI Grant Number JP19K20724 to N.K.
Institutional Review Board Statement
The present study was approved by the Etics committee, University of Toyama (Reference number: R2020025, date of approval: 1 May 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be provided from corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest associated with this manuscript.
Abbreviations
| CRT | cardiac resynchronization therapy |
| ECG | electrocardiogram |
| ICD | implantable cardioverter defibrillator |
| LBBB | left bundle branch block |
| LVEF | left ventricular ejection fraction |
| LVESV | left ventricular end-systolic volume |
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. American College of Cardiology Foundation. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Ezekowitz, J.A.; O’Meara, E.; McDonald, M.A.; Abrams, H.; Chan, M.; Ducharme, A.; Giannetti, N.; Grzeslo, A.; Hamilton, P.G.; Heckman, G.A.; et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017, 33, 1342–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockburger, M.; Moss, A.J.; Klein, H.U.; Zareba, W.; Goldenberg, I.; Biton, Y.; McNitt, S.; Kutyifa, V. Sustained clinical benefit of cardiac resynchronization therapy in non-LBBB patients with prolonged PR-interval: MADIT-CRT long-term follow-up. Clin. Res. Cardiol. 2016, 105, 944–952. [Google Scholar] [CrossRef]
- Steffel, J.; Robertson, M.; Singh, J.P.; Abraham, W.T.; Bax, J.J.; Borer, J.S.; Dickstein, K.; Ford, I.; Gorcsan, J.; Gras, D.; et al. The effect of QRS duration on cardiac resynchronization therapy in patients with a narrow QRS complex: A subgroup analysis of the EchoCRT trial. Eur. Heart J. 2015, 36, 1983–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparini, M.; Leclercq, C.; Yu, C.M.; Auricchio, A.; Steinberg, J.S.; Lamp, B.; Klersy, C.; Leyva, F. Absolute survival after cardiac resynchronization therapy according to baseline QRS duration: A multinational 10-year experience: Data from the Multicenter International CRT Study. Am. Heart J. 2014, 167, 203–209. [Google Scholar] [CrossRef]
- Brenyo, A.; Rao, M.; Barsheshet, A.; Cannom, D.; Quesada, A.; McNitt, S.; Huang, D.T.; Moss, A.J.; Zareba, W. QRS Axis and the Benefit of Cardiac Resynchronization Therapy in Patients with Mildly Symptomatic Heart Failure Enrolled in MADIT-CRT. J. Cardiovasc. Electrophysiol. 2013, 24, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Végh, E.M.; Kandala, J.; Januszkiewicz, L.; Ren, J.; Miller, A.; Orencole, M.; Blendea, D.; Merkely, B.; Gellér, L.; Singh, J.P.; et al. A new simplified electrocardiographic score predicts clinical outcome in patients treated with CRT. EP Eur. 2017, 20, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Kamath, S.A.; Neto, J.D.P.M.; Canham, R.M.; Uddin, F.; Toto, K.H.; Nelson, L.L.; Kaiser, P.A.; De Lemos, J.A.; Drazner, M.H. Low voltage on the electrocardiogram is a marker of disease severity and a risk factor for adverse outcomes in patients with heart failure due to systolic dysfunction. Am. Heart J. 2006, 152, 355–361. [Google Scholar] [CrossRef]
- Roberts, W.C.; Kondapalli, N.; Hall, S.A. Usefulness of total 12-lead QRS voltage for diagnosis of arrhythmogenic right ventricular cardiomyopathy in patients with heart failure severe enough to warrant orthotopic heart transplantation and morphologic illustration of its cardiac diversity. Am. J. Cardiol. 2018, 122, 1051–1061. [Google Scholar] [CrossRef]
- Roberts, W.C.; Becker, T.M.; Hall, S.A. Usefulness of total 12-lead QRS voltage as a clue to diagnosis of patients with cardiac sarcoidosis severe enough to warrant orthotopic heart transplant. JAMA Cardiol. 2018, 3, 64–68. [Google Scholar] [CrossRef]
- Nagase, S.; Kamakura, T.; Kataoka, N.; Wada, M.; Yamagata, K.; Ishibashi, K.; Inoue, Y.Y.; Miyamoto, K.; Noda, T.; Aiba, T.; et al. Low-Voltage Type 1 ECG Is Associated with Fatal Ventricular Tachyarrhythmia in Brugada Syndrome. J. Am. Heart Assoc. 2018, 7, e009713. [Google Scholar] [CrossRef] [Green Version]
- Gerber, B.L.; Rousseau, M.F.; Ahn, S.A.; Waroux, J.-B.L.P.D.; Pouleur, A.-C.; Phlips, T.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.-L. Prognostic Value of Myocardial Viability by Delayed-Enhanced Magnetic Resonance in Patients with Coronary Artery Disease and Low Ejection Fraction: Impact of Revascularization Therapy. J. Am. Coll. Cardiol. 2012, 59, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Höke, U.; Khidir, M.J.; van der Geest, R.J.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Relation of myocardial contrast-enhanced T1 mapping by cadiac magnetic resonance to left ventricular reverse remodeling after cardiac resynchronization therapy in patients with nonishchemic cardiomyopathy. Am. J. Cardiol. 2017, 119, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sohal, M.; Sammut, E.; Child, N.; Jackson, T.; Claridge, S.; Cooklin, M.; O’neill, M.; Wright, M.; Gill, J.; et al. Focal but not diffuse myocardial fibrosis burden quantification using cardiac mgnetic resonance imaging predicts left ventricular reverse modeling following cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2016, 27, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Stellbrink, C.; Breithardt, O.-A.; Franke, A.; Sack, S.; Bakker, P.; Auricchio, A.; Pochet, T.; Salo, R.; Kramer, A.; Spinelli, J. Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J. Am. Coll. Cardiol. 2001, 38, 1957–1965. [Google Scholar] [CrossRef] [Green Version]
- Bašinskas, P.; Stoškutė, N.; Gerulytė, A.; Abramavičiūtė, A.; Puodžiukynas, A.; Kazakevičius, T. Prognostication of Poor Survival after Cardiac Resynchronization Therapy. Medicina 2020, 56, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipahi, I.; Chou, J.C.; Hyden, M.; Rowland, D.Y.; Simon, D.I.; Fang, J.C. Effect of QRS morphology on clinical event reduction with cardiac resynchronization therapy: Meta-analysis of randomized controlled trials. Am. Heart J. 2012, 163, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Ypenburg, C.; van Bommel, R.; Borleffs, C.J.W.; Bleeker, G.B.; Boersma, E.; Schalij, M.J.; Bax, J.J. Long-Term Prognosis After Cardiac Resynchronization Therapy Is Related to the Extent of Left Ventricular Reverse Remodeling at Midterm Follow-Up. J. Am. Coll. Cardiol. 2009, 53, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Sipahi, I.; Carrigan, T.P.; Rowland, D.Y.; Stambler, B.S.; Fang, J.C. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: Meta-analysis of randomized controlled trials. Arch. Intern. Med. 2011, 171, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Stavrakis, S.; Lazzara, R.; Thadani, U. The benefit of cardiac resynchronization therapy and QRS duration: A meta-analysis. J. Cardiovasc. Electrophysiol. 2012, 23, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kutyifa, V.; Stockburger, M.; Daubert, J.P.; Holmqvist, F.; Olshansky, B.; Schuger, C.; Klein, H.; Goldenberg, I.; Brenyo, A.; McNitt, S.; et al. PR interval identifies clinical response in patients with non-left bundle branch block: A Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy substudy. Circ. Arrhythm. Electrophysiol. 2014, 7, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; Caso, P.; Romano, S.; Scarafile, R.; Riegler, L.; Salerno, G.; Limongelli, G.; Di Salvo, G.; Calabrò, P.; Del Viscovo, L.; et al. Different effects of cardiac resynchronization therapy on left atrial function in patients with either idiopathic or ischaemic dilated cardiomyopathy: A two-dimensional speckle strain study. Eur. Heart J. 2007, 28, 2738–2748. [Google Scholar] [CrossRef] [PubMed]
- Badran, H.A.; Abdelhamid, M.A.; Ibrahim, M.T.; Abdelmoteleb, A.M.; Zarif, J.K. Left atrium in cardiac resynchronization therapy: Active participant or innocent bystander. J. Saudi Heart Assoc. 2017, 29, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, L.; Kandala, J.; Di Biase, L.; Valleggi, A.; Michelotti, F.; Pieragnoli, P.; Ricciardi, G.; Mascioli, G.; Lakkireddy, D.; Pillarisetti, J.; et al. Prognostic Impact of QRS Axis Deviation in Patients Treated with Cardiac Resynchronization Therapy. J. Cardiovasc. Electrophysiol. 2016, 27, 315–320. [Google Scholar] [CrossRef]
- Fabiszak, T.; Łach, P.; Ratajczak, J.; Koziński, M.; Krupa, W.; Kubica, J. Influence of QRS duration and axis on response to cardiac resynchronization therapy in chronic heart failure with reduced left ventricular ejection fraction: A single center study including patients with left bundle branch block. Cardiol. J. 2020, 27, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).