An In Vitro Anti-Cancer Activity of Ocimum tenuiflorum Essential Oil by Inducing Apoptosis in Human Gastric Cancer Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of O. tenuiflorum Essential Oil (OTEO)
2.2. Cell Culture
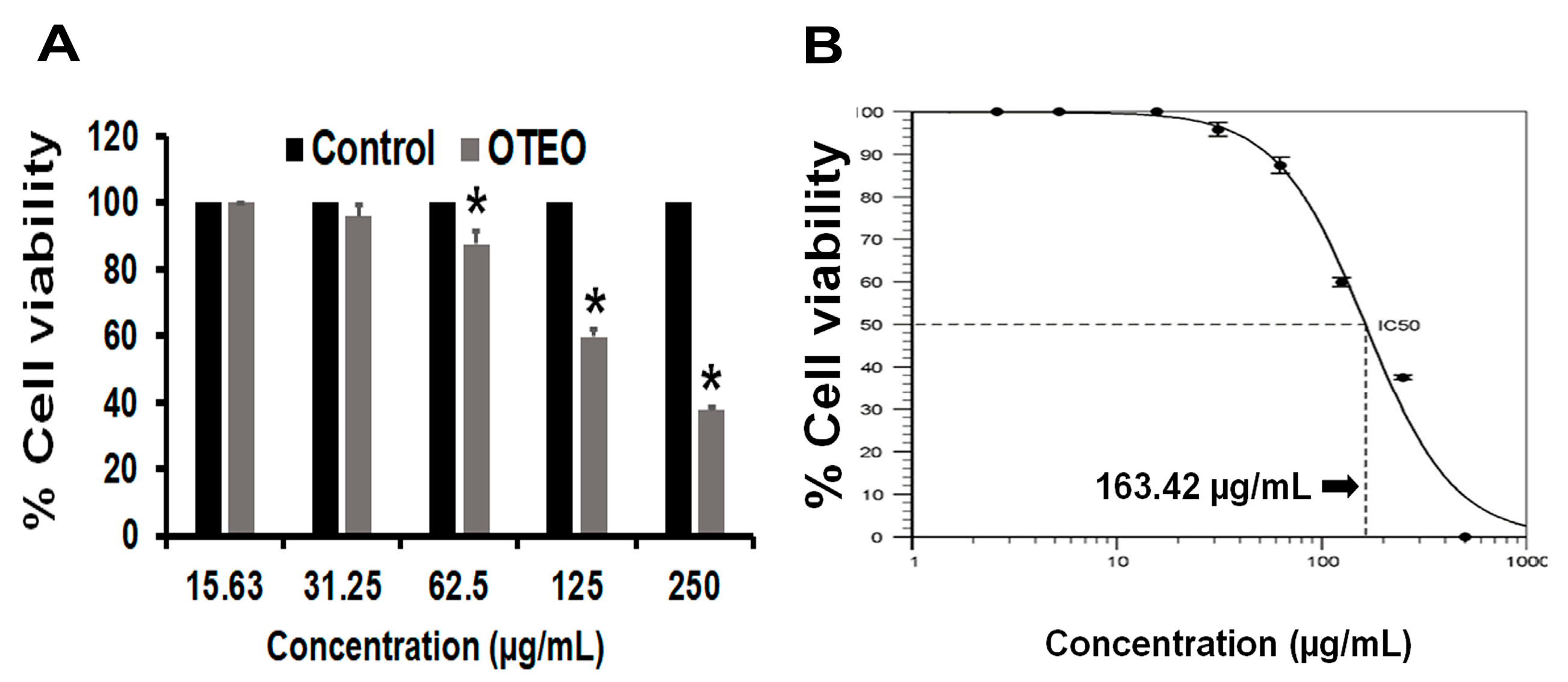
2.3. Determination of Cell Viability
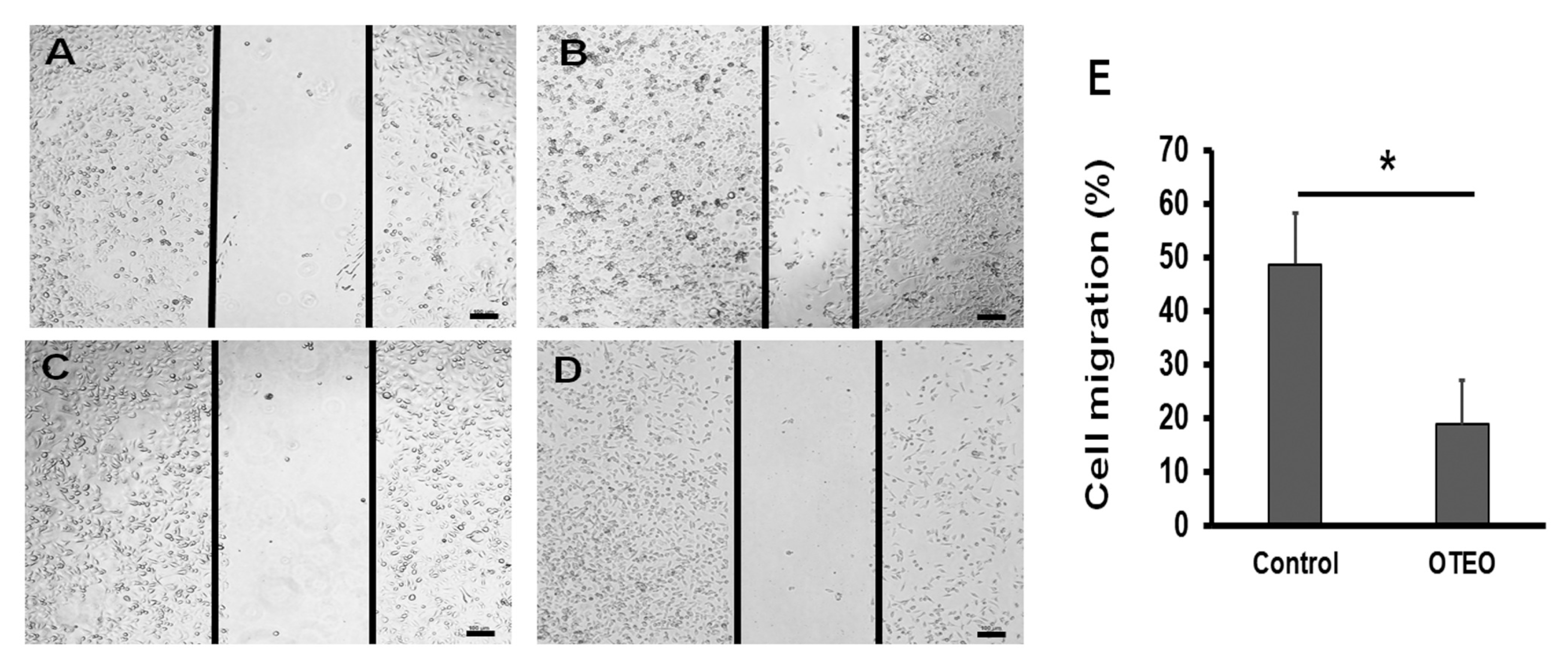
2.4. Cell Migration Assay
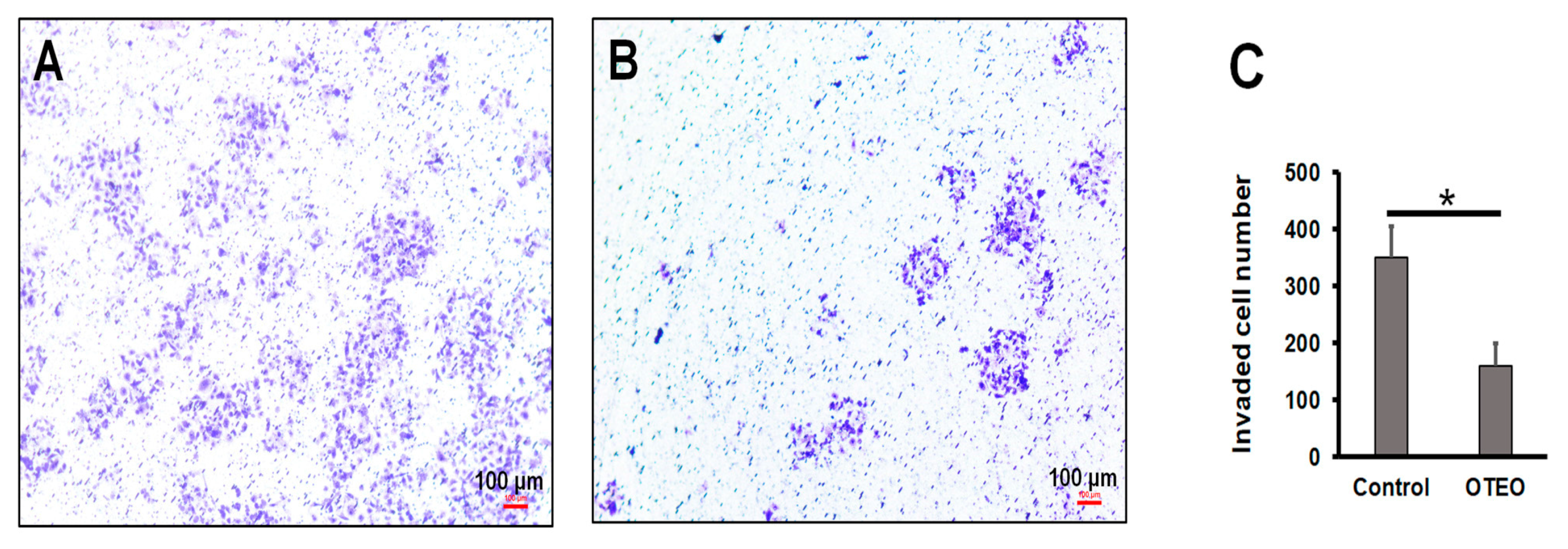
2.5. Cell Invasion Assay
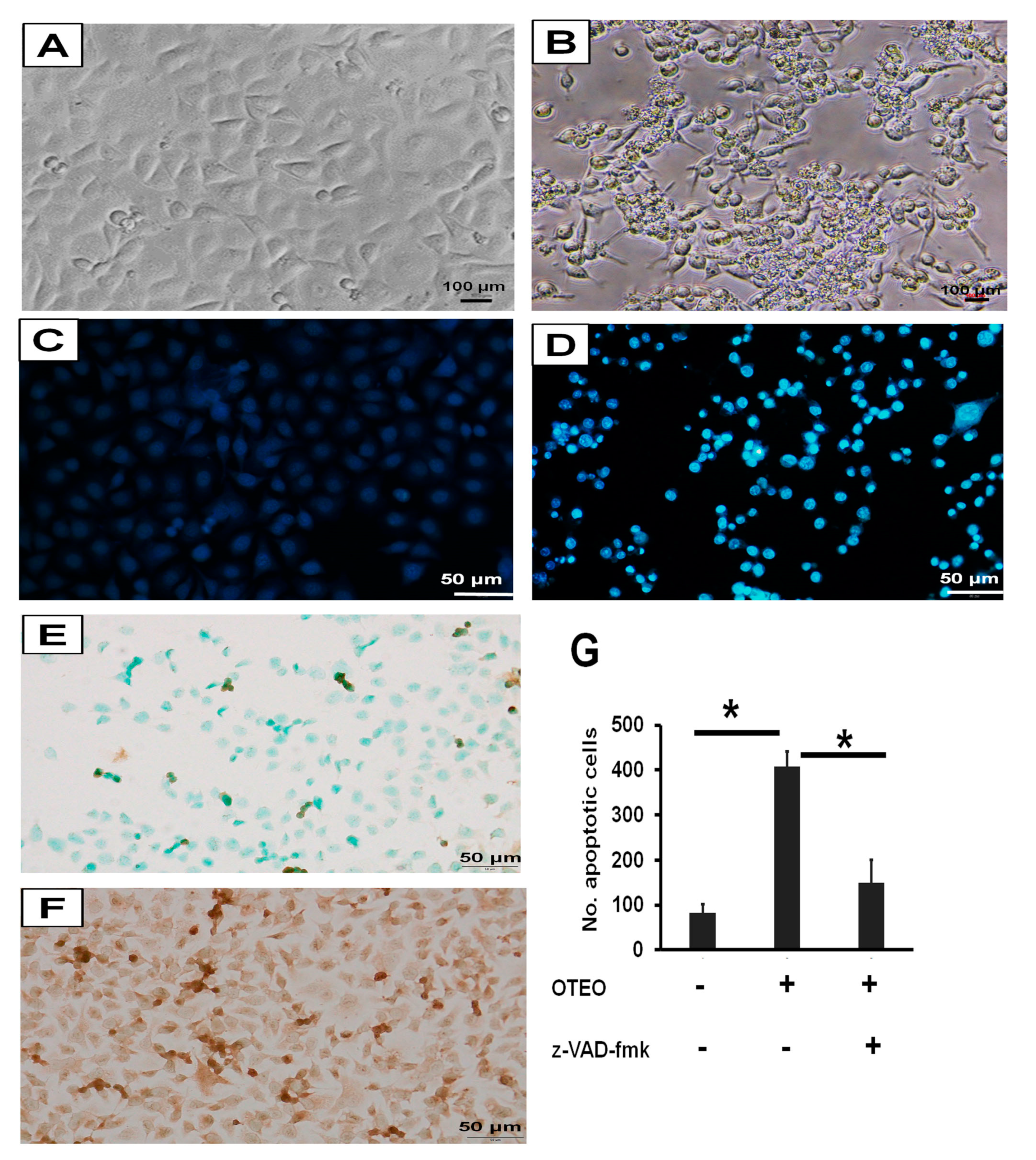
2.6. Determination of Cell Death and Nuclear Morphology
2.7. Apoptosis-Related Gene Expression by Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.8. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
2.9. Statistical Analysis
3. Results
3.1. OTEO Inhibits AGS Cell Viability
3.2. OTEO Inhibits Migration and Invasion of AGS Cells
3.3. Characterization of Cell Death and Nuclear Morphology in AGS Cells
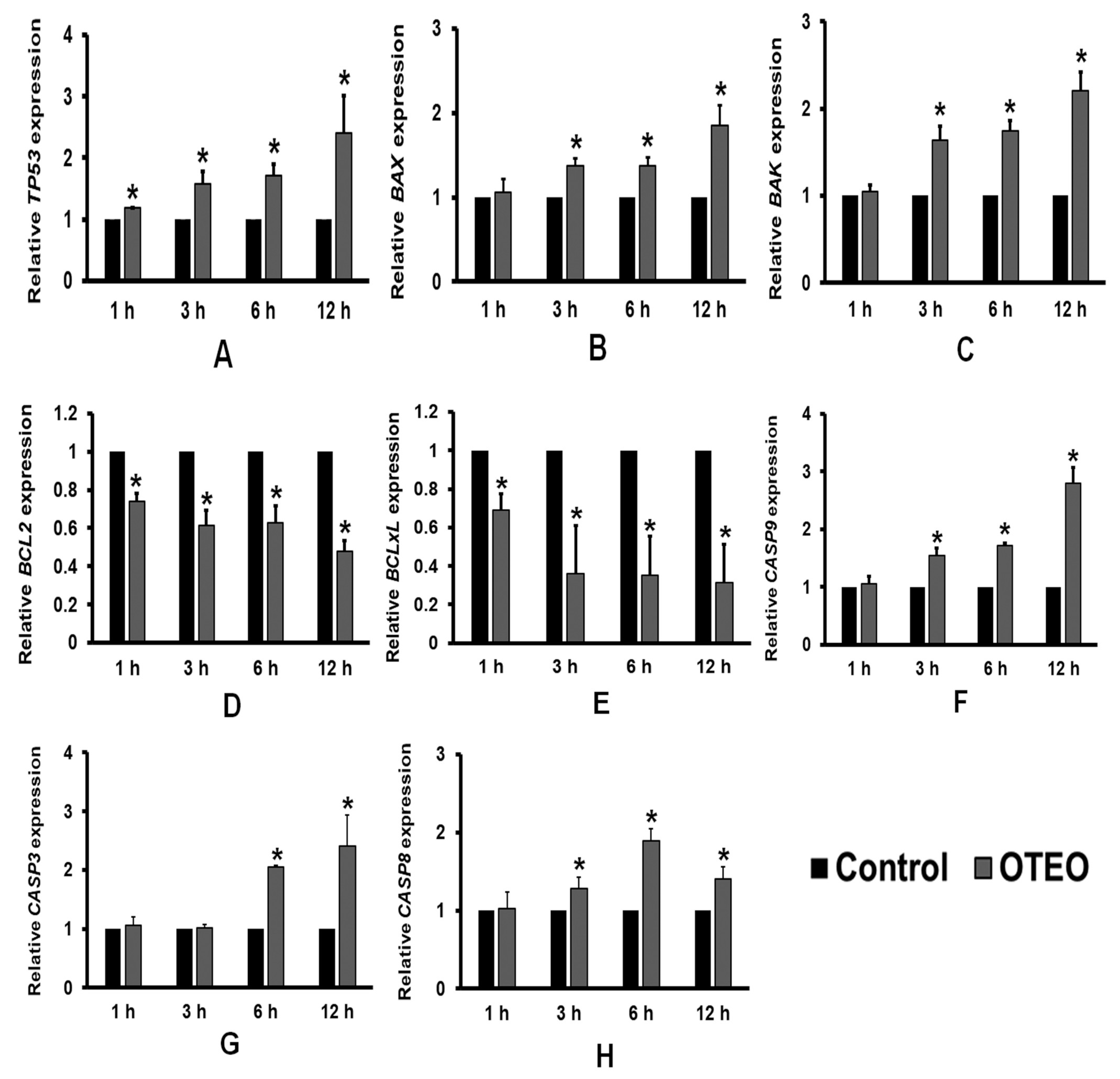
3.4. Effects of OTEO on Apoptosis-Related Gene Expression in AGS Cells
3.5. Chemical Composition of OTEO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Shin, A.; Kim, J.; Park, S. Gastric cancer epidemiology in Korea. J. Gastric Cancer 2011, 11, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.J.; Gao, J.B. Molecular mechanisms of chemoresistance in gastric cancer. World J. Gastrointest. Oncol. 2016, 8, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Casaretto, L.; Sousa, P.L.; Mari, J.J. Chemotherapy versus support cancer treatment in advanced gastric cancer: A meta-analysis. Braz. J. Med. Biol. Res. 2006, 39, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Wohrer, S.S.; Raderer, M.; Hejna, M. Palliative chemotherapy for advanced gastric cancer. Ann. Oncol. 2004, 15, 1585–1595. [Google Scholar] [CrossRef]
- Sastre, J.; Garcia-Saenz, J.A.; Diaz-Rubio, E. Chemotherapy for gastric cancer. World J. Gastroenterol. 2006, 12, 204–213. [Google Scholar] [CrossRef]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S.K. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhandayuthapani, S.; Azad, H.; Rathinavelu, A. Apoptosis Induction by Ocimum sanctum Extract in LNCaP Prostate Cancer Cells. J. Med. Food 2015, 18, 776–785. [Google Scholar] [CrossRef]
- Magesh, V.; Lee, J.C.; Ahn, K.S.; Lee, H.J.; Lee, H.J.; Lee, E.O.; Shim, B.S.; Jung, H.J.; Kim, J.S.; Kim, D.K.; et al. Ocimum sanctum induces apoptosis in A549 lung cancer cells and suppresses the in vivo growth of Lewis lung carcinoma cells. Phytother. Res. 2009, 23, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhaes, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Alternat. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Manaharan, T.; Thirugnanasampandan, R.; Jayakumar, R.; Kanthimathi, M.S.; Ramya, G.; Ramnath, M.G. Purified Essential Oil from Ocimum sanctum Linn. Triggers the Apoptotic Mechanism in Human Breast Cancer Cells. Pharmacogn. Mag. 2016, 12, S327–S331. [Google Scholar] [CrossRef]
- Namwat, N.; Amimanan, P.; Loilome, W.; Jearanaikoon, P.; Sripa, B.; Bhudhisawasdi, V.; Tassaneeyakul, W. Characterization of 5-fluorouracil-resistant cholangiocarcinoma cell lines. Chemotherapy 2008, 54, 343–351. [Google Scholar] [CrossRef]
- Jafari, N.; Zargar, S.J.; Yassa, N.; Delnavazi, M.R. Induction of Apoptosis and Cell Cycle Arrest by Dorema Glabrum Root Extracts in a Gastric Adenocarcinoma (AGS) Cell Line. Asian Pac. J. Cancer Prev. 2016, 17, 5189–5193. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dai, L.; Luo, L.; Xu, W.; Zhang, C.; Zhu, Y.; Chen, Z.; Hu, W.; Xu, X.; Pan, W. Non-essential amino acids attenuate apoptosis of gastric cancer cells induced by glucose starvation. Oncol. Rep. 2014, 32, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soutto, M.; Chen, Z.; Saleh, M.A.; Katsha, A.; Zhu, S.; Zaika, A.; Belkhiri, A.; El-Rifai, W. TFF1 activates p53 through down-regulation of miR-504 in gastric cancer. Oncotarget 2014, 5, 5663–5673. [Google Scholar] [CrossRef] [Green Version]
- Karaliotas, G.I.; Mavridis, K.; Scorilas, A.; Babis, G.C. Quantitative analysis of the mRNA expression levels of BCL2 and BAX genes in human osteoarthritis and normal articular cartilage: An investigation into their differential expression. Mol. Med. Rep. 2015, 12, 4514–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borhani, N.; Manoochehri, M.; Gargari, S.S.; Novin, M.G.; Mansouri, A.; Omrani, M.D. Decreased Expression of Proapoptotic Genes Caspase-8-and BCL2-Associated Agonist of Cell Death (BAD) in Ovarian Cancer. Clin. Ovarian Other Gynecologic. Cancer 2014, 7, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Mane, S.D.; Thoh, M.; Sharma, D.; Sandur, S.K.; Naidu, K.A. Ascorbyl Stearate Promotes Apoptosis Through Intrinsic Mitochondrial Pathway in HeLa Cancer Cells. Anticancer Res. 2016, 36, 6409–6417. [Google Scholar] [CrossRef]
- Friedl, P.; Hegerfeldt, Y.; Tusch, M. Collective cell migration in morphogenesis and cancer. Int. J. Dev. Biol. 2004, 48, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Rong, Z.; Huang, C. Surgery Strategies for Gastric Cancer with Liver Metastasis. Front. Oncol. 2019, 9, 1353. [Google Scholar] [CrossRef] [Green Version]
- Sonoda, H.; Kawai, K.; Yamaguchi, H.; Murono, K.; Kaneko, M.; Nishikawa, T.; Otani, K.; Sasaki, K.; Yasuda, K.; Tanaka, T.; et al. Lymphogenous metastasis to the transverse colon that originated from signet-ring cell gastric cancer: A case report and review of the literature. Clin. Res. Hepatol. Gastroenterol. 2017, 41, e81–e86. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Akiyama, H.; Atari, M.; Fukuhara, M.; Nakajima, Y.; Kinosita, H.; Uramoto, H. Pulmonary Resection for Metastatic Gastric Cancer. Ann. Thorac. Cardiovasc. Surg. 2016, 22, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Zhu, Z.; Shi, Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull. Cancer 2014, 101, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Wihadmadyatami, H.; Karnati, S.; Hening, P.; Tjahjono, Y.; Maharjanti, F.; Kusindarta, D.L.; Triyono, T. Ethanolic extract Ocimum sanctum Linn. induces an apoptosis in human lung adenocarcinoma (A549) cells. Heliyon 2019, 5, e02772. [Google Scholar] [CrossRef] [PubMed]
- Utispan, K.; Niyomtham, N.; Yingyongnarongkul, B.E.; Koontongkaew, S. Ethanolic Extract of Ocimum sanctum Leaves Reduced Invasion and Matrix Metalloproteinase Activity of Head and Neck Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2020, 21, 363–370. [Google Scholar] [CrossRef]
- Kwak, T.K.; Sohn, E.J.; Kim, S.; Won, G.; Choi, J.U.; Jeong, K.; Jeong, M.; Kwon, O.S.; Kim, S.H. Inhibitory effect of ethanol extract of Ocimum sanctum on osteopontin mediated metastasis of NCI-H460 non-small cell lung cancer cells. BMC Complement. Altern. Med. 2014, 14, 419. [Google Scholar] [CrossRef] [Green Version]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaccia, A.J.; Kastan, M.B. The complexity of p53 modulation: Emerging patterns from divergent signals. Genes Dev. 1998, 12, 2973–2983. [Google Scholar] [CrossRef] [Green Version]
- Haupt, S.; Berger, M.; Goldberg, Z.; Haupt, Y. Apoptosis—The p53 network. J. Cell Sci. 2003, 116, 4077–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinds, M.G.; Day, C.L. Regulation of apoptosis: Uncovering the binding determinants. Curr. Opin. Struct. Biol. 2005, 15, 690–699. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.M.; Cory, S. Bcl-2-regulated apoptosis: Mechanism and therapeutic potential. Curr. Opin. Immunol. 2007, 19, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangpao, T.; Chung, H.H.; Sommano, S.R. Aromatic Profiles of Essential Oils from Five Commonly Used Thai Basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkan, A.; Erdoğan, A. A comparative evaluation of antioxidant and anticancer activity of essential oil from origanum onites (lamiaceae) and its two major phenolic components. Turk. J. Biol. 2011, 35, 735–742. [Google Scholar] [CrossRef]
| Gene | Primers | Annealing (°C) |
|---|---|---|
| GAPDH | Forward: 5′-TCATCAGCAATGCCTCCTGCA-3′ | 55 |
| Reverse: 5′-TGGGTGGCAGTGATGGCA-3′ | ||
| BCL-2 | Forward: 5′-CAGGATAACGGAGGCTGGGATG-3′ | 60 |
| Reverse: 5′-AGAAATCAAACAGAGGCCGCA-3′ | ||
| BCL-xL | Forward: 5′-ACCCCAGGGACAGCATATCA-3′ | 60 |
| Reverse: 5′-TGCGATCCGACTCACCAATA-3′ | ||
| TP53 | Forward: 5′-TAACAGTTCCTGCATGGGCGGC-3′ | 55 |
| Reverse: 5′-AGGACAGGCACAAACACGCACC-3′ | ||
| BAX | Forward: 5′-TGGCAGCTGACATGTTTTCTGAC-3′ | 60 |
| Reverse: 5′-TCACCCAACCACCCTGGTCTT-3′ | ||
| BAK | Forward: 5′-ATGGTCACCTTACCTCTGCAA-3′ | 60 |
| Reverse: 5′-TCATAGCGTCGGTTGATGTCG-3′ | ||
| CASP8 | Forward: 5′-AGAGTCTGTGCCCAAATCAAC-3′ | 60 |
| Reverse: 5′-GCTGCTTCTCTCTTTGCTGAA-3′ | ||
| CASP9 | Forward: 5′-CGAACTAACAGGCAAGCAGC-3′ | 60 |
| Reverse: 5′-ACCTCACCAAATCCTCCAGAAC-3′ | ||
| CASP3 | Forward: 5′-GCGGTTGTAGAAGAGTTTCGTG-3′ | 60 |
| Reverse: 5′-CTCACGGCCTGGGATTTCAA-3′ |
| Retention Time (min) | Compounds | %Area |
|---|---|---|
| 4.61 | 2,5-Diethyltetrahydrofuran | 0.95 |
| 5.22 | 3-Carene | 0.53 |
| 5.42 | α-Pinene | 11.66 |
| 5.81 | Camphene | 9.37 |
| 6.31 | β-Phellandrene | 1.54 |
| 6.46 | β-Pinene | 5.96 |
| 6.66 | β-Myrcene | 0.69 |
| 7.65 | O-Cymene | 1.60 |
| 7.79 | D-Limonene | 3.11 |
| 7.90 | Eucalyptol | 8.26 |
| 8.23 | β-Ocimene | 1.86 |
| 8.63 | γ-Terpinene | 0.39 |
| 9.82 | Linalyl acetate | 3.01 |
| 11.35 | Camphor | 1.21 |
| 11.90 | Isobornyl thiocyanoacetate | 0.99 |
| 12.15 | Isoborneol | 2.03 |
| 12.48 | γ-Terpinene | 0.40 |
| 12.90 | α-Terpineol | 2.03 |
| 12.97 | Estragole | 3.28 |
| 17.95 | Eugenol | 4.70 |
| 18.76 | Copaene | 0.88 |
| 19.17 | β-Elemene | 4.68 |
| 19.44 | Methyl eugenol | 4.25 |
| 20.18 | Caryophyllene | 25.85 |
| 21.22 | Humulene | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonyanugomol, W.; Rukseree, K.; Prapatpong, P.; Reamtong, O.; Baik, S.-C.; Jung, M.; Shin, M.-K.; Kang, H.-L.; Lee, W.-K. An In Vitro Anti-Cancer Activity of Ocimum tenuiflorum Essential Oil by Inducing Apoptosis in Human Gastric Cancer Cell Line. Medicina 2021, 57, 784. https://doi.org/10.3390/medicina57080784
Boonyanugomol W, Rukseree K, Prapatpong P, Reamtong O, Baik S-C, Jung M, Shin M-K, Kang H-L, Lee W-K. An In Vitro Anti-Cancer Activity of Ocimum tenuiflorum Essential Oil by Inducing Apoptosis in Human Gastric Cancer Cell Line. Medicina. 2021; 57(8):784. https://doi.org/10.3390/medicina57080784
Chicago/Turabian StyleBoonyanugomol, Wongwarut, Kamolchanok Rukseree, Pornpan Prapatpong, Onrapak Reamtong, Seung-Chul Baik, Myunghwan Jung, Min-Kyoung Shin, Hyung-Lyun Kang, and Woo-Kon Lee. 2021. "An In Vitro Anti-Cancer Activity of Ocimum tenuiflorum Essential Oil by Inducing Apoptosis in Human Gastric Cancer Cell Line" Medicina 57, no. 8: 784. https://doi.org/10.3390/medicina57080784






