Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults
Abstract
1. Introduction
2. Materials and Methods
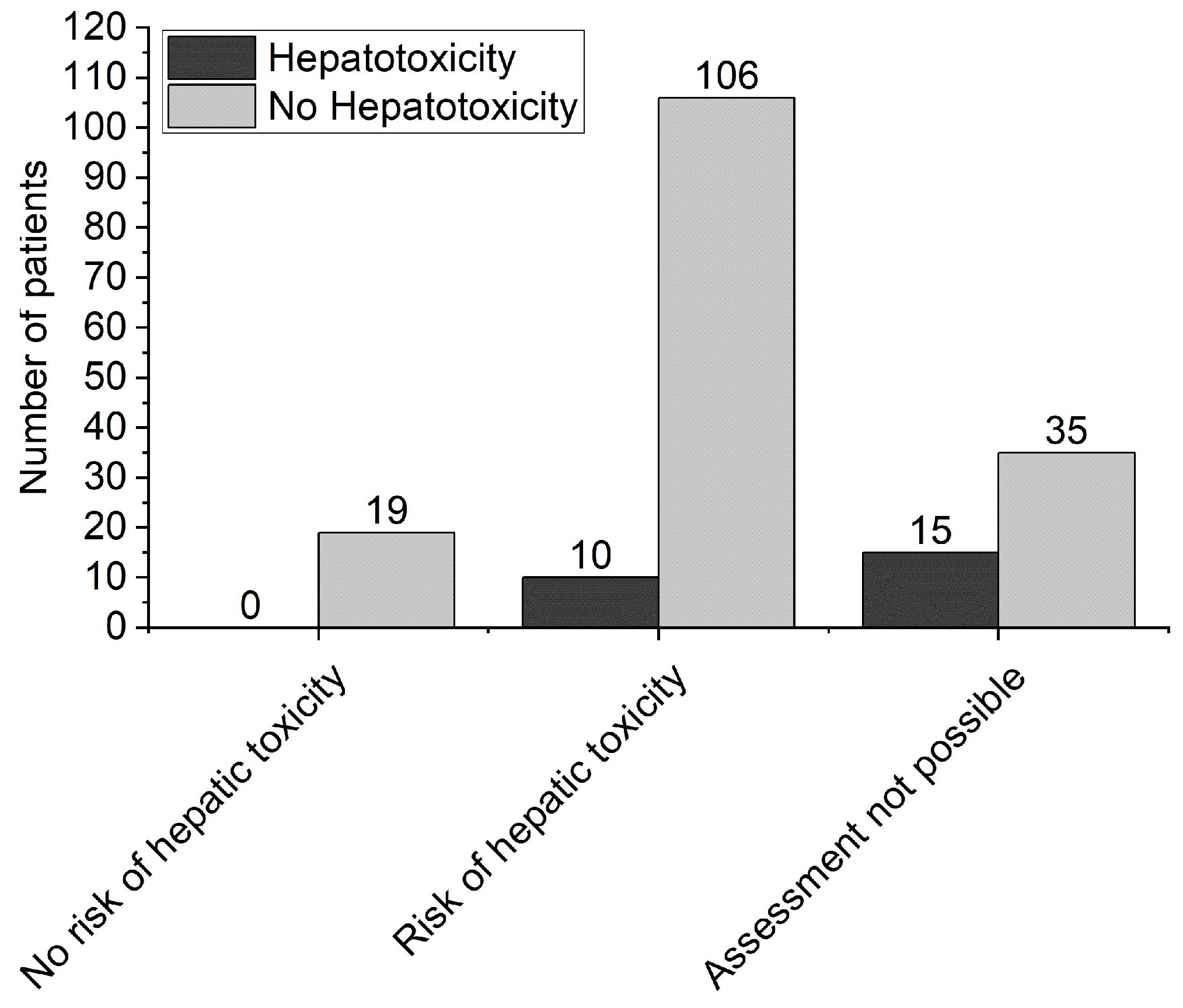
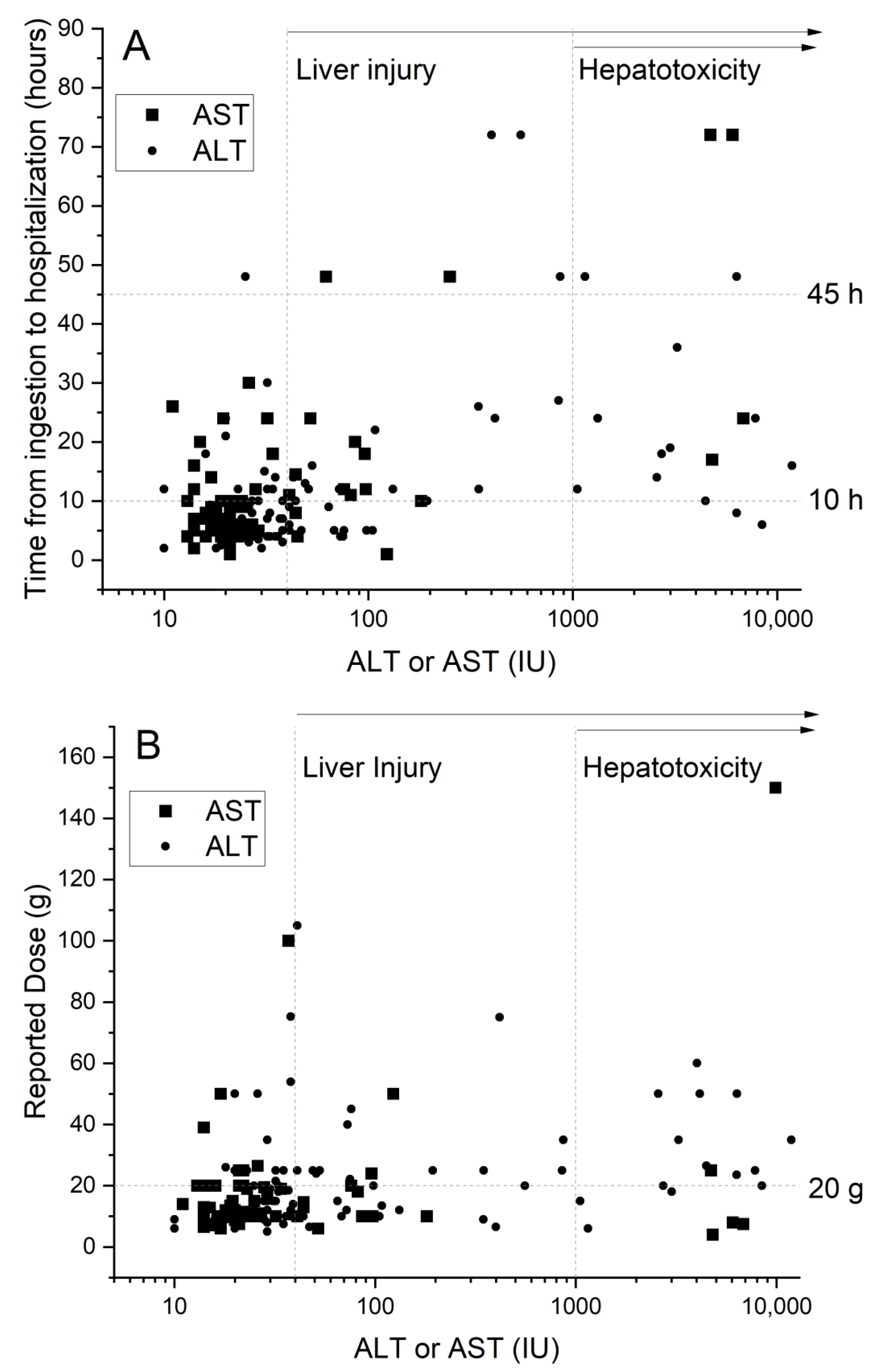
3. Results
3.1. Patient Characteristics
3.2. Comparison between the Groups
3.3. Logistic Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lancaster, E.M.; Hiatt, J.R.; Zarrinpar, A. Acetaminophen hepatotoxicity: An updated review. Arch. Toxicol. 2015, 89, 193–199. [Google Scholar] [CrossRef]
- Marks, D.J.B.; Dargan, P.; Archer, J.R.H.; Davies, C.L.; Dines, A.M.; Wood, D.; Greene, S.L. Outcomes from massive paracetamol overdose: A retrospective observational study: Massive paracetamol overdose. Br. J. Clin. Pharmacol. 2017, 83, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guideline Panel. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef]
- Popiołek, I.; Piotrowicz-Wójcik, K.; Porebski, G. Hypersensitivity Reactions in Serious Adverse Events Reported for Paracetamol in the EudraVigilance Database, 2007–2018. Pharmacy 2019, 7, 12. [Google Scholar] [CrossRef]
- Rumack, B.H.; Matthew, H. Acetaminophen poisoning and toxicity. Pediatrics 1975, 55, 871–876. [Google Scholar]
- White, S.J.; Rumack, B.H. The Acetaminophen Toxicity Equations: “Solutions” for Acetaminophen Toxicity Based on the Rumack-Matthew Nomogram. Ann. Emerg. Med. 2005, 45, 563–564. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Dinant, V.; Ruggiano, I.; Willem, H.; Laterre, P.-F.; Hantson, P. Pattern of Paracetamol Poisoning: Influence on Outcome and Complications. Toxics 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Sivilotti, M.; Graudins, A. Accuracy of the paracetamol-aminotransferase multiplication product to predict hepatotoxicity in modified-release paracetamol overdose. Clin. Toxicol. 2017, 55, 346–351. [Google Scholar] [CrossRef]
- Chomchai, S.; Chomchai, C.; Anusornsuwan, T. Acetaminophen psi parameter: A useful tool to quantify hepatotoxicity risk in acute acetaminophen overdose. Clin. Toxicol. 2011, 49, 664–667. [Google Scholar] [CrossRef]
- Wong, A.; Graudins, A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin. Toxicol. 2017, 55, 879–892. [Google Scholar] [CrossRef]
- Waring, W.S.; Stephen, A.; Robinson, O.; Dow, M.; Pettie, J. Serum urea concentration and the risk of hepatotoxicity after paracetamol overdose. QJM Int. J. Med. 2008, 101, 359–363. [Google Scholar] [CrossRef]
- Weissenborn, K. Hepatic Encephalopathy: Definition, Clinical Grading and Diagnostic Principles. Drugs 2019, 79, 5–9. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; Clinical Practice Guidelines Panel; Wendon, J.; Cordoba, J.; Dhawan, A.; Larsen, F.S.; Manns, M.; Nevens, F.; Samuel, D.; Simpson, K.J.; et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017, 66, 1047–1081. [Google Scholar] [CrossRef]
- O’Grady, J.G.; Alexander, G.J.; Hayllar, K.M.; Williams, R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989, 97, 439–445. [Google Scholar] [CrossRef]
- Chomchai, S.; Chomchai, C. Predicting acute acetaminophen hepatotoxicity with acetaminophen-aminotransferase multiplication product and the Psi parameter. Clin. Toxicol. 2014, 52, 506–511. [Google Scholar] [CrossRef]
- Pettie, J.M.; Caparrotta, T.M.; Hunter, R.W.; Morrison, E.E.; Wood, D.M.; Dargan, P.I.; Thanacoody, R.H.; Thomas, S.H.; Elamin, M.E.; Francis, B.; et al. Safety and Efficacy of the SNAP 12-hour Acetylcysteine Regimen for the Treatment of Paracetamol Overdose. EClinicalMedicine 2019, 11, 11–17. [Google Scholar] [CrossRef]
- Hawton, K.; Ware, C.; Mistry, H.; Hewitt, J.; Kingsbury, S.; Roberts, D.; Weitzel, H. Paracetamol Self-Poisoning Characteristics, Prevention and Harm Reduction. Br. J. Psychiatry 1996, 168, 43–48. [Google Scholar] [CrossRef]
- Myers, R.P.; Li, B.; Fong, A.; Shaheen, A.A.M.; Quan, H. Hospitalizations for acetaminophen overdose: A Canadian population-based study from 1995 to 2004. BMC Public Health 2007, 7, 143. [Google Scholar] [CrossRef]
- Chiew, A.L.; Gluud, C.; Brok, J.; Buckley, N.A. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst. Rev. 2018, 2018, CD003328. [Google Scholar] [CrossRef]
- Chomchai, S.; Chomchai, C. Being overweight or obese as a risk factor for acute liver injury secondary to acute acetaminophen overdose. Pharmacoepidemiol. Drug Saf. 2018, 27, 19–24. [Google Scholar] [CrossRef]
- Acheampong, P.; Thomas, S.H.L. Determinants of hepatotoxicity after repeated supratherapeutic paracetamol ingestion: Systematic review of reported cases. Br. J. Clin. Pharmacol. 2016, 82, 923–931. [Google Scholar] [CrossRef]
- Sivilotti, M.L.; Green, T.J.; Langmann, C.; Yarema, M.; Juurlink, D.; Johnson, D. Multiplying the serum aminotransferase by the acetaminophen concentration to predict toxicity following overdose. Clin. Toxicol. 2010, 48, 793–799. [Google Scholar] [CrossRef]
- Green, T.J.; Sivilotti, M.L.; Langmann, C.; Yarema, M.; Juurlink, D.; Burns, M.J.; Johnson, D.W. When do the aminotransferases rise after acute acetaminophen overdose? Clin. Toxicol. 2010, 48, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.G.N.; Ford, A.; Hayes, P.C.; Simpson, K.J. Systematic review: Prognostic tests of paracetamol-induced acute liver failure. Aliment. Pharmacol. Ther. 2010, 31, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Brok, J.; Buckley, N.; Gluud, C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst. Rev. 2006, 2, CD003328. [Google Scholar] [CrossRef]
- Green, J.L.; Heard, K.J.; Reynolds, K.M.; Albert, D. Oral and Intravenous Acetylcysteine for Treatment of Acetaminophen Toxicity: A Systematic Review and Meta-analysis. West. J. Emerg. Med. 2013, 14, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.E. Age and paracetamol self-poisoning. Gut 2005, 54, 686–690. [Google Scholar] [CrossRef][Green Version]
- Rumack, B.H. Acetaminophen Hepatotoxicity: The First 35 Years. J. Toxicol. Clin. Toxicol. 2002, 40, 3–20. [Google Scholar] [CrossRef]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [CrossRef]
| Group A (129 Patients, 92/37 F/M) | Group B (31 Patients, 15/16 F/M) | Group C (25 Patients, 9/16 F/M) | p | |
|---|---|---|---|---|
| Laboratory parameters | ||||
| ALT, IU/L | 19 (10) | 137 (231) | 2464 (2657) * | <0.001 |
| AST, IU/L | 20 (8) | 83 (99) | 3669 (5675) * | <0.001 |
| [PRC]pl, mg/L | 101.1 (105.2) | 76.3 (78.5) | 102 (104.3) | 0.57 |
| [PRC]4h, mg/L | 169 (153) | 239 (322) | 373 (249) * | 0.11 |
| Bilirubin, µmol/L | 13 (10) | 16 (10) | 67 (117) * | <0.001 |
| INR | 1.15 (0.15) | 1.14 (0.14) | 1.79 (0.77) | <0.001 |
| Platelet count, 103/mL | 240 (60) | 250 (62) | 181 (67) * | <0.001 |
| Hemoglobin, g/dL | 13.1 (1.7) | 13.3 (1.9) | 13.2 (2.2) | 0.70 |
| Leukocyte count, 103/µL | 8 (3.2) | 9.1 (3.5) | 8 (3.2) | 0.39 |
| Creatinine, µmol/L | 64 (19) | 67 (16) | 117 (168) | 0.16 |
| Clinical features | ||||
| Paracetamol dose, grams | 17.00 (15.58) | 23.12 (15.44) * | 31.50 (31.45) * | <0.001 |
| Time from ingestion to hospital admission, hours | 8 (7) | 20 (19) * | 30 (22) * | <0.001 |
| Age, years | 28 (15) | 30 (14) | 32 (15) | 0.40 |
| BMI, kg/m2 | 23.4 (4.5) | 25.6 (5.8) | 25 (5) | 0.15 |
| Variables | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR | 95% CI | Adjusted OR | 95% CI | |
| Male gender | 2.94 | 1.24–6.99 | 1.85 | 0.35–9.88 |
| Alcohol abuse | 3.55 | 1.46–8.61 | 3.59 | 0.69–18.66 |
| Age, years | 1.01 | 0.99–1.04 | 0.99 | 0.94–1.05 |
| BMI, kg/m2 | 1.05 | 0.96–1.15 | 1.04 | 0.88–1.23 |
| Paracetamol dose, grams | 1.03 | 1.01–1.05 | 1.02 | 0.98–1.07 |
| Time from ingestion to hospital presentation, hours | 1.06 | 1.03–1.09 | 1.08 | 1.03–1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiolek, I.; Hydzik, P.; Jagielski, P.; Zrodlowska, M.; Mystek, K.; Porebski, G. Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults. Medicina 2021, 57, 752. https://doi.org/10.3390/medicina57080752
Popiolek I, Hydzik P, Jagielski P, Zrodlowska M, Mystek K, Porebski G. Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults. Medicina. 2021; 57(8):752. https://doi.org/10.3390/medicina57080752
Chicago/Turabian StylePopiolek, Iwona, Piotr Hydzik, Pawel Jagielski, Monika Zrodlowska, Karol Mystek, and Grzegorz Porebski. 2021. "Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults" Medicina 57, no. 8: 752. https://doi.org/10.3390/medicina57080752
APA StylePopiolek, I., Hydzik, P., Jagielski, P., Zrodlowska, M., Mystek, K., & Porebski, G. (2021). Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults. Medicina, 57(8), 752. https://doi.org/10.3390/medicina57080752







