On the Track of New Endoscopic Alternatives for the Treatment of Selected Gastric GISTs—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group Characteristics
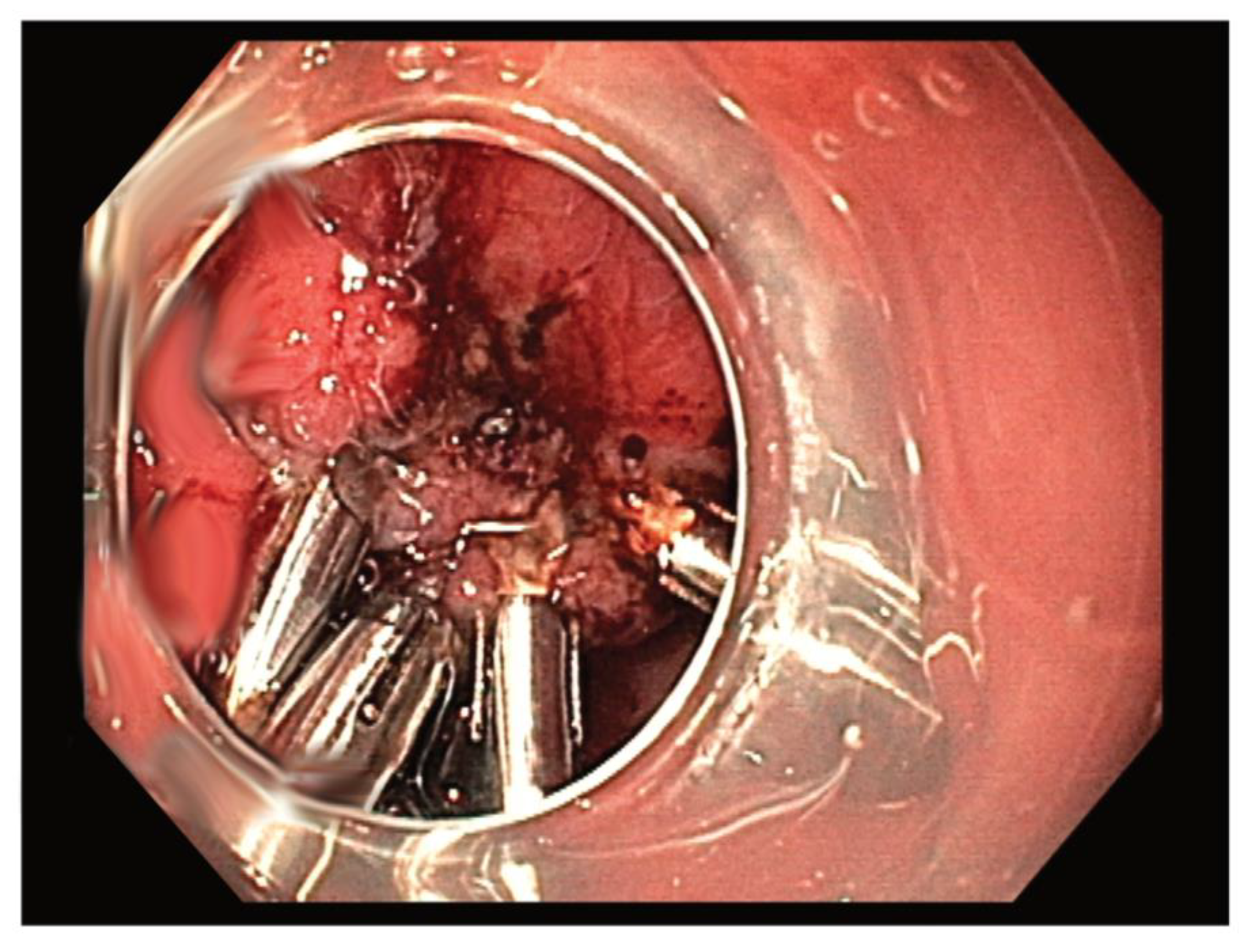
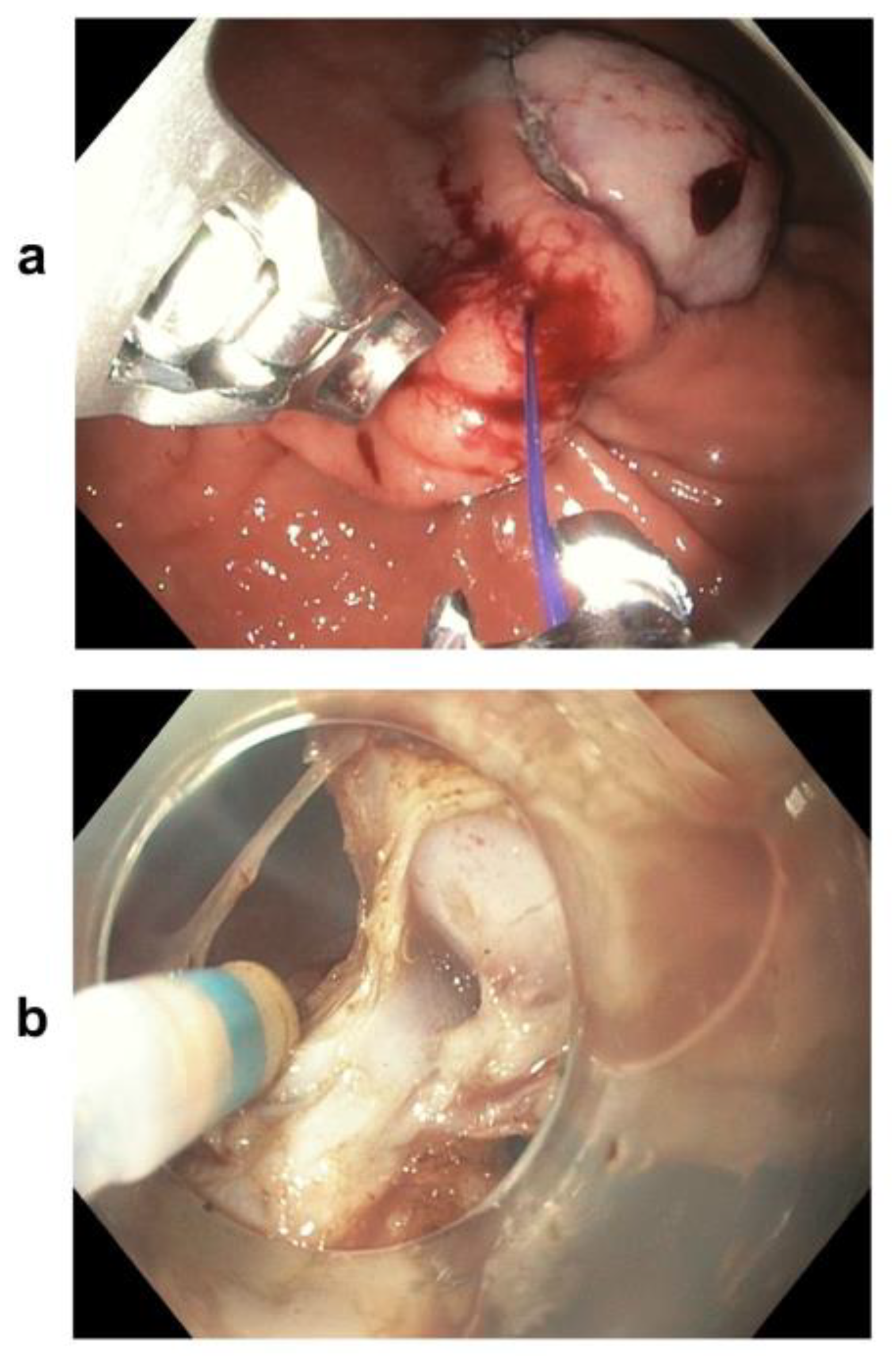
2.2. Resection Techniques
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
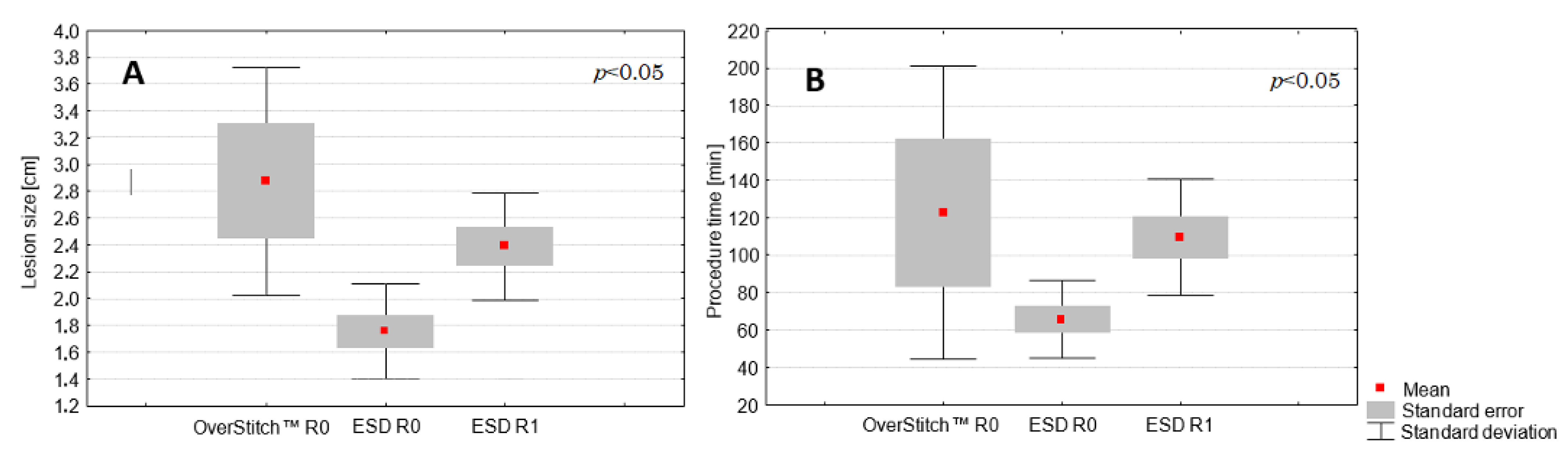
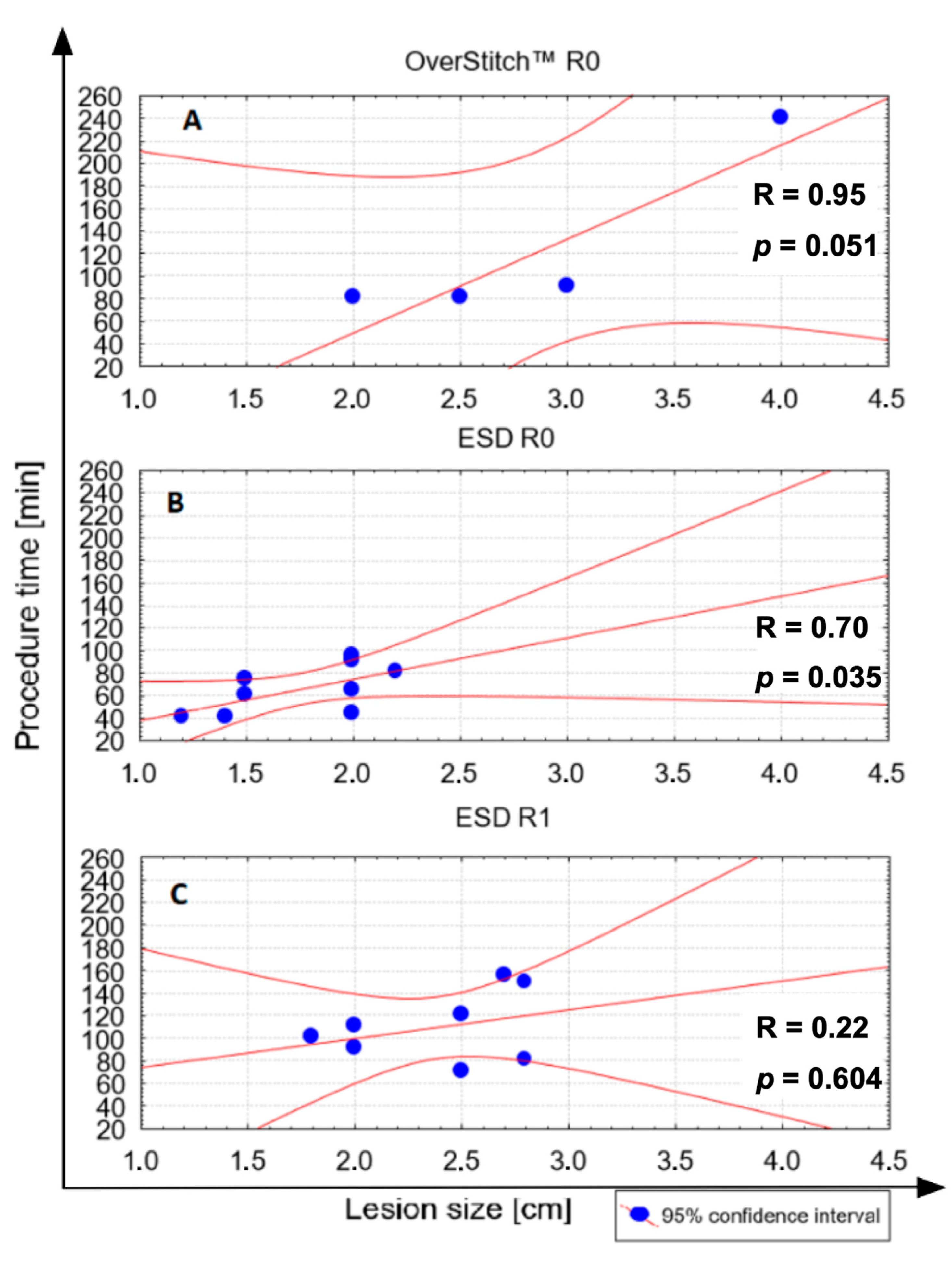
3.2. Comparison between ESD and the Hybrid Technique
4. Discussion
5. Conclusions
6. What Is Known
- -
- Gastrointestinal stromal tumours (GISTs) are the most common mesenchymal lesions of the gastrointestinal tract, constituting nearly 80% of mesenchymal pathologies.
- -
- Due to possible malignant transformation and unexpected, rapid growth, close surveillance and early excision are the mainstay of management.
- -
- The less invasive procedure includes various endoscopic resection techniques, such as ESD, EFTR, and STER.
7. What We Found
- -
- The hybrid technique combining endoscopic resection and endoluminal suturing appears to be a potential alternative for selected gastric GISTs with a large size and a high MP connection grade (type > I), with advantages over ESD.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burch, J.; Ahmad, I. Gastrointestinal Stromal Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554541/ (accessed on 9 July 2020).
- Yoo, I.K.; Cho, J.Y. Endoscopic Treatment for Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract. Clin. Endosc. 2020, 53, 383–384. [Google Scholar] [CrossRef]
- Nishida, T.; Hirota, S.; Yanagisawa, A.; Sugino, Y.; Minami, M.; Yamamura, Y.; Otani, Y.; Shimada, Y.; Takahashi, F.; Kubota, T. Clinical practice guidelines for gastrointestinal stromal tumour (GIST) in Japan: English version. Int. J. Clin. Oncol. 2008, 13, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.; Abecassis, N.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; Brodowicz, T.; Broto, J.; et al. Gastrointestinal stromal tumours: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv68–iv78. [Google Scholar] [CrossRef]
- Rutkowski, P.; Skoczylas, J.; Wisniewski, P. Is the Surgical Margin in Gastrointestinal Stromal Tumors Different? Visc. Med. 2018, 34, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Park, S. Laparoscopic techniques and strategies for gastrointestinal GISTs. J. Vis. Surg. 2017, 3, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcella, C.; Shi, R.H.; Sarwar, S. Clinical Overview of GIST and Its Latest Management by Endoscopic Resection in Upper GI: A Literature Review. Gastroenterol. Res. Pract. 2018, 2018, 6864256. [Google Scholar] [CrossRef] [Green Version]
- Akahoshi, K.; Oya, M.; Koga, T.; Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World J. Gastroenterol. 2018, 24, 2806–2817. [Google Scholar] [CrossRef]
- von Mehren, M.; Kane, J.M.; Bui, M.M.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J. Natl. Compr. Canc. Netw. 2020, 18, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H. Endoscopic treatment for gastrointestinal stromal tumor: Advantages and hurdles. World J. Gastrointest. Endosc. 2015, 7, 192–205. [Google Scholar] [CrossRef]
- Pawlak, K.M.; Raiter, A.; Kozłowska-Petriczko, K.; Szełemej, J.; Petriczko, J.; Wojciechowska, K.; Wiechowska-Kozłowska, A. Optimal Endoscopic Resection Technique for Selected Gastric GISTs. The Endoscopic Suturing System Combined with ESD—a New Alternative? J. Clin. Med. 2020, 9, 1776. [Google Scholar] [CrossRef]
- An, W.; Sun, P.-B.; Gao, J.; Jiang, F.; Liu, F.; Chen, J.; Wang, D.; Li, Z.-S.; Shi, X.-G. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: A retrospective cohort study. Surg. Endosc. 2017, 31, 4522–4531. [Google Scholar] [CrossRef]
- Lee, I.L.; Lin, P.Y.; Tung, S.Y.; Shen, C.-H.; Wei, K.L.; Wu, C.S. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tu-mours originating from the muscularis propria layer. Endoscopy 2006, 38, 1024–1028. [Google Scholar] [CrossRef]
- Białek, A.; Wiechowska-Kozłowska, A.; Pertkiewicz, J.; Polkowski, M.; Milkiewicz, P.; Karpińska, K.; Ławniczak, M.; Starzyńska, T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest. Endosc. 2012, 75, 276–286. [Google Scholar] [CrossRef]
- Shichijo, S.; Uedo, N.; Yanagimoto, Y.; Yamamoto, K.; Kono, M.; Fukuda, H.; Shimamoto, Y.; Nakagawa, K.; Ohmori, M.; Arao, M.; et al. Endoscopic full-thickness resection of gastric gastrointestinal stromal tumor: A Japanese case series. Ann. Gastroenterol. 2019, 32, 593–599. [Google Scholar] [CrossRef]
- Santos-Antunes, J.; Baldaque-Silva, F.; Marques, M.; Lopes, J.; Carneiro, F.; Macedo, G. Real-life evaluation of the safety, efficacy and therapeutic outcomes of endoscopic submucosal dissection in a Western tertiary centre. United Eur. Gastroenterol. J. 2018, 6, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Yao, L.Q.; Zhou, P.H.; Xu, M.D.; Chen, S.Y.; Zhong, Y.S.; Zhang, Y.Q.; Chen, W.F.; Ma, L.L.; Qin, W.Z. Submucosal tumours of the esophagogastric junction originating from the muscularis pro-pria layer: A large study of endoscopic submucosal dissection (with video). Gastrointest. Endosc. 2012, 75, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-R.; Song, J.-T.; Qu, B.; Wen, J.-F.; Yin, J.-B.; Liu, W. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg. Endosc. 2012, 26, 3141–3148. [Google Scholar] [CrossRef]
- Balde, A.I.; Chen, T.; Hu, Y.; Liu, H.; Gong, W.; Yu, J.; Zhen, L.; Li, G. Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: A propensity score-matched study. Surg. Endosc. 2016, 31, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.C.; Kim, J.H.; Kim, J.H.; Shen, J.Y.; Shen, K.M.; Shen, S.W. Endoscopic resection for the treatment of gastric subepithelial tumours originated from the muscularis propria layer. Hepatogastroenterology 2009, 56, 1281–1286. [Google Scholar]
- Białek, A.; Wiechowska-Kozłowska, A.; Pertkiewicz, J.; Karpińska, K.; Marlicz, W.; Milkiewicz, P.; Starzyńska, T. Endoscopic submucosal dissection for the treatment of neoplastic lesions in the gastrointestinal tract. World J. Gastroenterol. 2013, 19, 1953–1961. [Google Scholar] [CrossRef]
- Hulagu, S.; Senturk, O.; Aygün, C.; Kocaman, O.; Çelebi, A.; Konduk, T.; Koc, D.; Sirin, G.; Korkmaz, U.; Duman, A.E.; et al. Endoscopic submucosal dissection for premalignant lesions and noninvasive early gastrointestinal cancers. World J. Gastroenterol. 2011, 17, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Schmidt, A.; Glaser, N.; Meining, A.; Walter, B.; Wannhoff, A.; Riecken, B.; Caca, K. Endoscopic full-thickness resection of gastric subepithelial tumours with the gFTRD-system: A prospective pilot study (RESET trial). Surg. Endosc. 2020, 34, 853–860. [Google Scholar] [CrossRef]
- Pang, T.; Zhao, Y.; Fan, T.; Hu, Q.; Raymond, D.; Cao, S.; Xu, G. Comparison of Safety and Outcomes between Endoscopic and Surgical Resections of Small (≤ 5 cm) Primary Gastric Gastrointestinal Stromal Tumors. J. Cancer. 2019, 10, 4132–4141. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, K.; Chennubhotla, S.; Bhandari, B.; Arora, A.; Singhal, S. Closing the Gaps: Endoscopic Suturing for Large Submucosal and Full-Thickness Defects. Clin. Endosc. 2018, 51, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Rajan, E.; Song, L.M.W.K. Endoscopic Full Thickness Resection. Gastroenterol. 2018, 154, 1925–1937.e2. [Google Scholar] [CrossRef]
| Overall (n = 21) | ESD (n = 17) | OverStitch (n = 4) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (mean; SD) | 70 (8) | 70.5 (8.8) | 68 (6.5) | >0.20 |
| Female (n; %) | 10 (48%) | 9 (53%) | 1 (25%) | 0.58 |
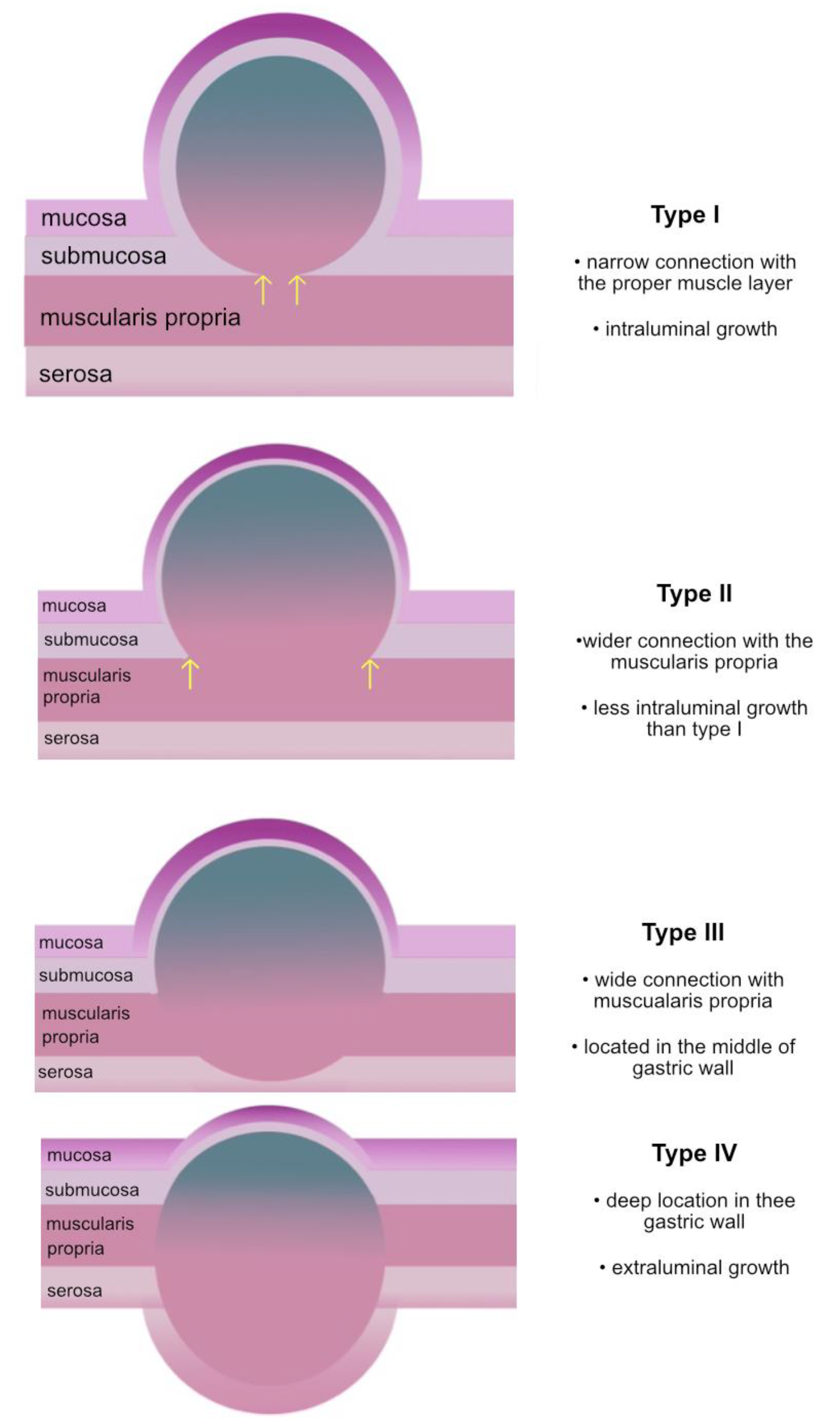
| Lesion type (n; %) | ||||
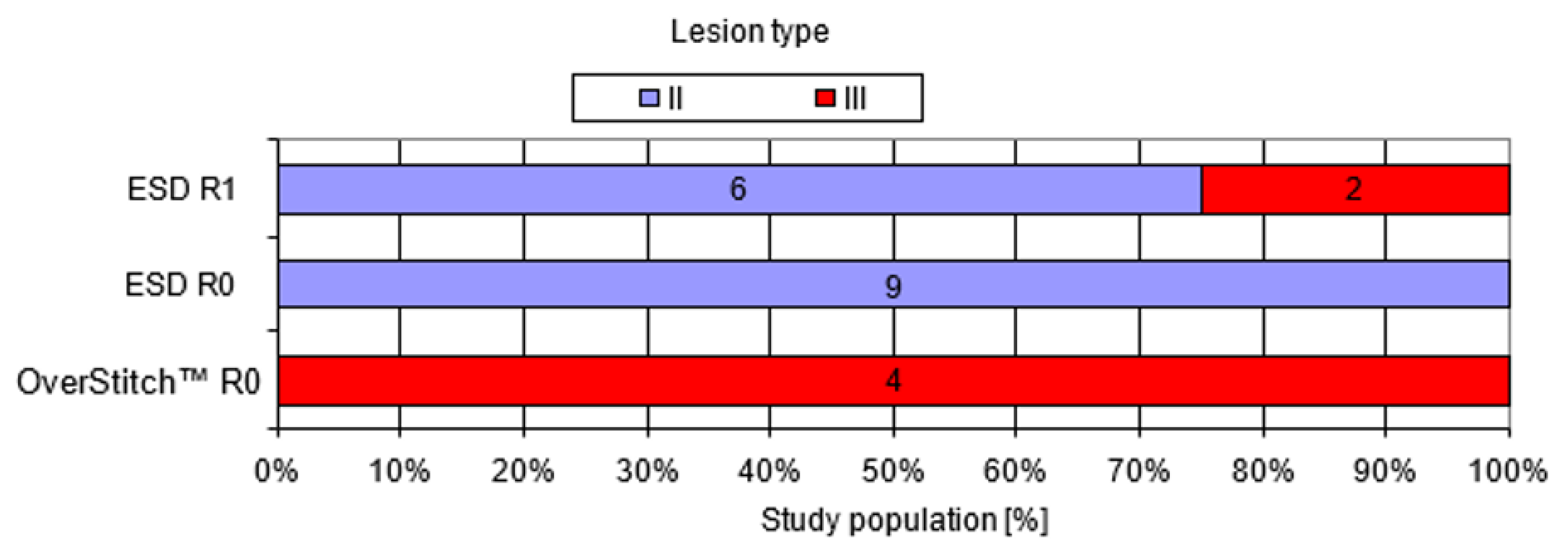
| II | 15 (71%) | 15 (88%) | 0 (0%) | <0.005 * |
| III | 6 (29%) | 2 (12%) | 4 (100%) | |
| Lesion localisation (n; %) | ||||
| Body | 12 (57%) | 11 (65%) | 1 (25%) | <0.01 * |
| Antrum | 7 (33%) | 6 (35%) | 1 (25%) | |
| Fundus | 2 (10%) | 0 (0%) | 2 (50%) | |
| Procedural aspects | ||||
| R0 resection (n; %) | 13 (62%) | 9 (53%) | 4 (100%) | 0.13 |
| Procedure time (min) (mean; SD) | 93.1 (45.35) | 86.2 (33.9) | 122.5 (78.5) | 0.15 |
| Lesion size (cm) (mean; SD) | 2.21 (0.64) | 2.05 (0.49) | 2.88 (0.85) | 0.016 * |
| Path result | Confirmed GIST < 5 mitoses/50 HPF (very low risk) | |||
| Adverse events rate (%) | 0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiter, A.; Pawlak, K.M.; Kozłowska-Petriczko, K.; Petriczko, J.; Szełemej, J.; Wiechowska-Kozłowska, A. On the Track of New Endoscopic Alternatives for the Treatment of Selected Gastric GISTs—A Pilot Study. Medicina 2021, 57, 625. https://doi.org/10.3390/medicina57060625
Raiter A, Pawlak KM, Kozłowska-Petriczko K, Petriczko J, Szełemej J, Wiechowska-Kozłowska A. On the Track of New Endoscopic Alternatives for the Treatment of Selected Gastric GISTs—A Pilot Study. Medicina. 2021; 57(6):625. https://doi.org/10.3390/medicina57060625
Chicago/Turabian StyleRaiter, Artur, Katarzyna M. Pawlak, Katarzyna Kozłowska-Petriczko, Jan Petriczko, Joanna Szełemej, and Anna Wiechowska-Kozłowska. 2021. "On the Track of New Endoscopic Alternatives for the Treatment of Selected Gastric GISTs—A Pilot Study" Medicina 57, no. 6: 625. https://doi.org/10.3390/medicina57060625
APA StyleRaiter, A., Pawlak, K. M., Kozłowska-Petriczko, K., Petriczko, J., Szełemej, J., & Wiechowska-Kozłowska, A. (2021). On the Track of New Endoscopic Alternatives for the Treatment of Selected Gastric GISTs—A Pilot Study. Medicina, 57(6), 625. https://doi.org/10.3390/medicina57060625






