Protective Effects of Quercetin on Oxidative Stress-Induced Tubular Epithelial Damage in the Experimental Rat Hyperoxaluria Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Hyperoxaluria-Induced Nephrolithiasis Model and Quercetin Administration
2.4. Biochemical Analyses
2.5. Histopathological Analyses
2.6. Immunohistochemical Analyses
2.7. Statistics
3. Results
3.1. Biochemical Results
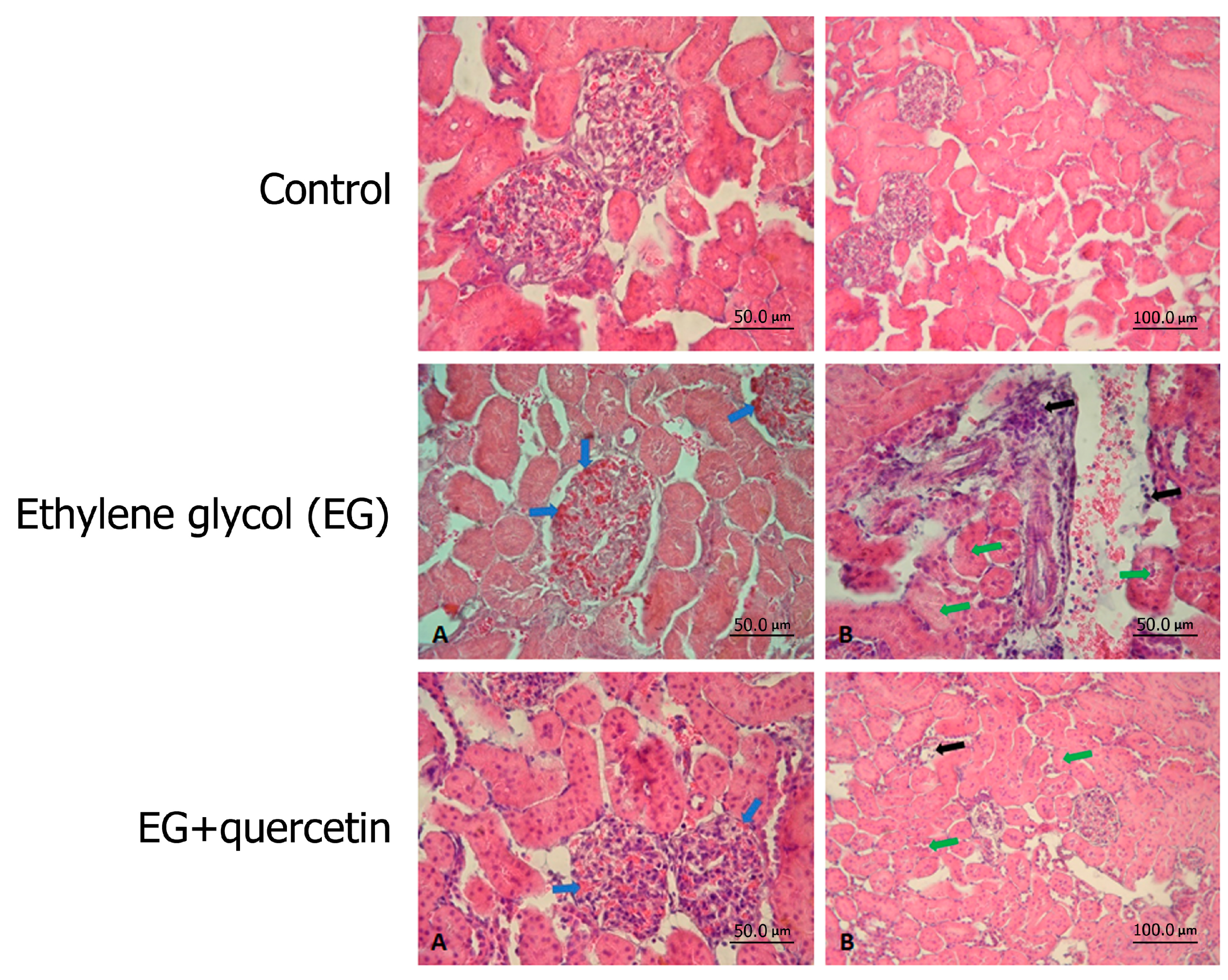
3.2. Histopathological Results
3.3. Immunohistochemical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gambaro, G.; Vezzoli, G.; Casari, G.; Rampoldi, L.; D’Angelo, A.; Borghi, L. Genetics of hypercalciuria and calcium nephrolithiasis: From the rare monogenic to the common polygenic forms. Am. J. Kidney Dis. 2004, 44, 963–986. [Google Scholar] [CrossRef]
- Coe, F.L.; Parks, J.H.; Asplin, J.H. The pathogenesis and treatment of kidney stones. N. Engl. J. Med. 1992, 327, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 16008. [Google Scholar] [CrossRef]
- Sun, X.Y.; Xu, M.; Ouyang, J.M. Effect of Crystal Shape and Aggregation of Calcium Oxalate Monohydrate on Cellular Toxicity in Renal Epithelial Cells. ACS Omega 2017, 2, 6039–6052. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, J.A.; Kohjimoto, Y.; Scheid, C.R.; Schmidt, M. Oxalate toxicity in renal cells. Urol. Res. 2005, 33, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Ryall, R.L.; Kavanagh, J.P.; Khan, S.R.; Kok, D.J.; Rodgers, A.L.; Tiselius, H.G. Methods for measuring crystallization in urolithiasis research: Why, how and when? Eur. Urol. 2001, 40, 220–230. [Google Scholar] [CrossRef]
- Menon, M.; Koul, H. Clinical review 32: Calcium oxalate nephrolithiasis. J. Clin. Endocrinol. Metab. 1992, 74, 703–707. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed]
- Abdelhalim, M.A.K.; Moussa, S.A.A.; Qaid, H.A.Y. The protective role of quercetin and arginine on gold nanoparticles induced hepatotoxicity in rats. Int. J. Nanomed. 2018, 13, 2821–2825. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, A.; Zal, F.; Bolouki, A. Protective effects of quercetin on nicotine induced oxidative stress in ‘HepG2 cells’. Toxicol. Mech. Methods 2017, 27, 609–614. [Google Scholar] [CrossRef]
- Huang, H.S.; Ma, M.C.; Chen, C.F.; Chen, J. Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology 2003, 62, 1123–1128. [Google Scholar] [CrossRef]
- Khan, S.R. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: Evidence from clinical and experimental investigations. J. Urol. 2013, 189, 803–811. [Google Scholar] [CrossRef]
- Evan, A.P.; Worcester, E.M.; Coe, F.L.; Williams, J., Jr.; Lingeman, J.E. Mechanisms of human kidney stone formation. Urolithiasis 2015, 43 (Suppl. 1), 19–32. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Nephrocalcinosis in animal models with and without stones. Urol. Res. 2010, 38, 429–438. [Google Scholar] [CrossRef]
- Ladwig, P.M.; Liedtke, R.R.; Larson, T.S.; Lieske, J.C. Sensitive spectrophotometric assay for plasma oxalate. Clin. Chem. 2005, 51, 2377–2380. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Method of Enzymatic Analysis, 2nd ed.; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–684. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Liu, H.S.; Pan, C.E.; Liu, Q.G.; Yang, W.; Liu, X.M. Effect of NF-kappaB and p38 MAPK in activated monocytes/macrophages on pro-inflammatory cytokines of rats with acute pancreatitis. World J. Gastroenterol. 2003, 9, 2513–2518. [Google Scholar] [CrossRef]
- Khan, S.R. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol. Res. 2005, 33, 349–357. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, Y.F.; Feng, Y.; Peng, B.; Che, J.P.; Liu, M.; Zheng, J.H. Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis 2014, 42, 519–526. [Google Scholar] [CrossRef]
- Ozturk, H.; Cetinkaya, A.; Firat, T.S.; Tekce, B.K.; Duzcu, S.E.; Ozturk, H. Protective effect of pentoxifylline on oxidative renal cell injury associated with renal crystal formation in a hyperoxaluric rat model. Urolithiasis 2019, 47, 415–424. [Google Scholar] [CrossRef]
- Gadge, N.B.; Jalalpure, S.S. Curative treatment with extracts of Bombax ceiba fruit reduces risk of calcium oxalate urolithiasis in rats. Pharm. Biol. 2012, 50, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B. Quercetin and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 377–387. [Google Scholar] [PubMed]
- Arts, M.J.T.J.; Dallinga, J.S.; Voss, H.P.; Haenen, G.R.M.M.; Bast, A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 2004, 88, 567–570. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Gautam, D.; Sharma, M.; Singla, S.K. Bergenin attenuates renal injury by reversing mitochondrial dysfunction in ethylene glycol induced hyperoxaluric rat model. Eur. J. Pharmacol. 2016, 791, 611–621. [Google Scholar] [CrossRef]
- Park, H.K.; Jeong, B.C.; Sung, M.K.; Park, M.Y.; Choi, E.Y.; Kim, B.S.; Kim, H.H.; Kim, J.I. Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J. Urol. 2008, 179, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ding, H.; Qin, Z.; Zhang, C.; Qi, S.; Zhang, H.; Yang, T.; He, Z.; Yang, K.; Du, E.; et al. Metformin Prevents Renal Stone Formation through an Antioxidant Mechanism In Vitro and In Vivo. Oxidative Med. Cell Longev. 2016, 2016, 4156075. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Thongboonkerd, V. p38 MAPK mediates calcium oxalate crystal-induced tight junction disruption in distal renal tubular epithelial cells. Sci. Rep. 2013, 3, 1041. [Google Scholar] [CrossRef]
- Qi, S.; Wang, Q.; Xie, B.; Chen, Y.; Zhang, Z.; Xu, Y. P38 MAPK signaling pathway mediates COM crystal-induced crystal adhesion change in rat renal tubular epithelial cells. Urolithiasis 2020, 48, 9–18. [Google Scholar] [CrossRef]

| Biochemical Variables | Control (n = 8) | EG (n = 8) | EG + Quercetin (n = 8) | p Values |
|---|---|---|---|---|
| MDA (nmol/mg protein) | 4.53 ± 0.89 a | 7.40 ± 1.64 b | 5.41 ± 0.82 a | 0.0001 |
| CAT (µmol/min/mg) | 118.41 ± 26.02 a | 119.48 ± 16.55 a | 131.06 ± 28.99 a | 0.360 |
| PUrea (mg/dL) | 42.37 ± 3.37 a | 86.75 ± 61.44 b | 51.62 ± 19.97 a | 0.040 |
| UCalcium (mg/dL) | 12.90 ± 3.37 a | 8.38 ± 3.39 b | 9.58 ± 5.59 b | 0.046 |
| Creatinine Clearance (mL/min) | 0.26 ± 0.09 a | 0.50 ± 0.28 a | 0.42 ± 0.25 a | 0.174 |
| POxalate (µmol/L) | 32.18 ± 19.28 a | 71.92 ± 61.83 a | 47.48 ± 20.09 a | 0.117 |
| UOxalate (µmol/24 h) | 2.72 ± 0.36 a | 10.14 ± 4.13 b | 6.81 ± 2.26 c | 0.0001 |
| Immunohistochemical Variables | Comparison of Groups Control and EG | Comparison of Groups Control and EG + Quercetin | Comparison of Groups EG and EG + Quercetin |
|---|---|---|---|
| p38-MAPK | p < 0.001 | p > 0.036 | p < 0.004 |
| NF-kB | p < 0.001 | p > 0.227 | p > 0.054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzel, A.; Yunusoglu, S.; Calapoglu, M.; Candan, I.A.; Onaran, I.; Oncu, M.; Ergun, O.; Oksay, T. Protective Effects of Quercetin on Oxidative Stress-Induced Tubular Epithelial Damage in the Experimental Rat Hyperoxaluria Model. Medicina 2021, 57, 566. https://doi.org/10.3390/medicina57060566
Guzel A, Yunusoglu S, Calapoglu M, Candan IA, Onaran I, Oncu M, Ergun O, Oksay T. Protective Effects of Quercetin on Oxidative Stress-Induced Tubular Epithelial Damage in the Experimental Rat Hyperoxaluria Model. Medicina. 2021; 57(6):566. https://doi.org/10.3390/medicina57060566
Chicago/Turabian StyleGuzel, Ahmet, Sedat Yunusoglu, Mustafa Calapoglu, Ibrahim Aydın Candan, Ibrahim Onaran, Meral Oncu, Osman Ergun, and Taylan Oksay. 2021. "Protective Effects of Quercetin on Oxidative Stress-Induced Tubular Epithelial Damage in the Experimental Rat Hyperoxaluria Model" Medicina 57, no. 6: 566. https://doi.org/10.3390/medicina57060566
APA StyleGuzel, A., Yunusoglu, S., Calapoglu, M., Candan, I. A., Onaran, I., Oncu, M., Ergun, O., & Oksay, T. (2021). Protective Effects of Quercetin on Oxidative Stress-Induced Tubular Epithelial Damage in the Experimental Rat Hyperoxaluria Model. Medicina, 57(6), 566. https://doi.org/10.3390/medicina57060566






