Comparison of Anti-Bacterial Efficacy between Passive Ultrasonic Irrigation and 980 nm-GaAlAs Laser Application in Two Root Types
Abstract
1. Introduction
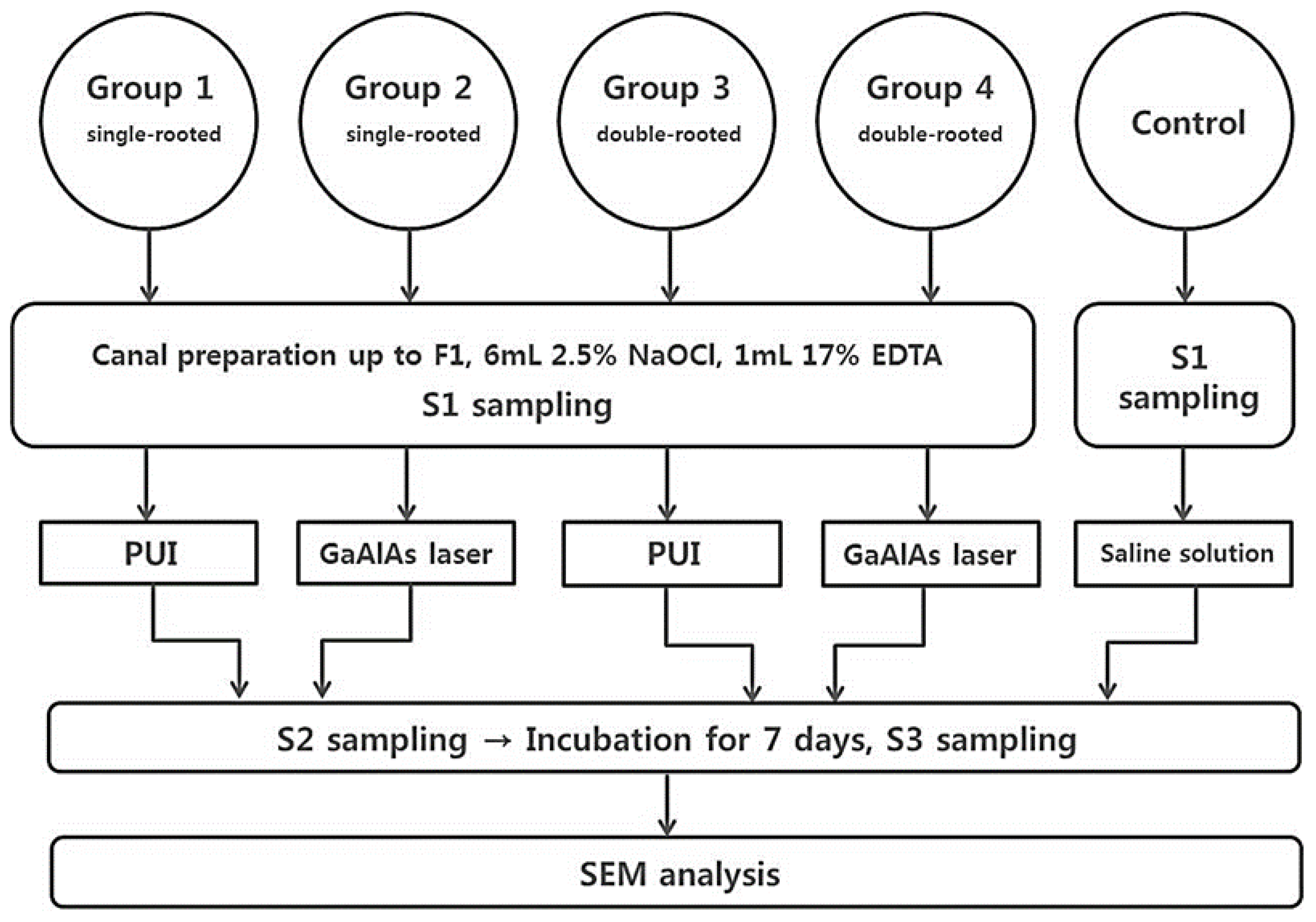
2. Materials and Methods
2.1. Sample Preparation
2.2. Bacterial Preparation and Inoculation
2.3. Classification of the Groups
- Single root + PUI group (n = 20): PUI was performed using a piezoelectric ultrasonic device at a power setting of 2 (Satelec, Acteon, Merignac, France) with an Irrisafe tip (15/K size, Acteon, Mt. Laurel, NJ, USA) in an in-and out motion for up to two-thirds of the length of the canal for a total of 1 min.
- Single root + laser application group (n = 20): Before irradiation with a GaAlAs diode laser (Hulaser, Seoul, Korea), the canal was fully dried with paper points (Dentsply Maillefer). Then, the canal was irradiated with a pulsed 980-nm GaAlAs diode laser at an output power of 1 W at 50 Hz. During the laser irradiation, a slow helical movement method was applied in the canal with a 200-μmdiameter fiber tip for 10 s and a 10-s break for up to two-thirds of the length of the canal for a total of 1 min.
- Double roots + PUI group (n = 20): The protocol was similar to group 1, except that each tooth in this group contained two root canals.
- Double roots + laser application group (n = 20): The protocol was similar to group 2, except that each tooth in this group contained two root canals.
- Control group (n = 3 single root, 3 double roots): The root canals were irrigated with sterile saline solution, not with 2.5% NaOCl.
2.4. Microbiological Analysis
2.5. DNA Extraction and qPCR Analysis
2.6. Preparation for Scanning Electron Microscopy
2.7. Statistical Analysis
3. Results
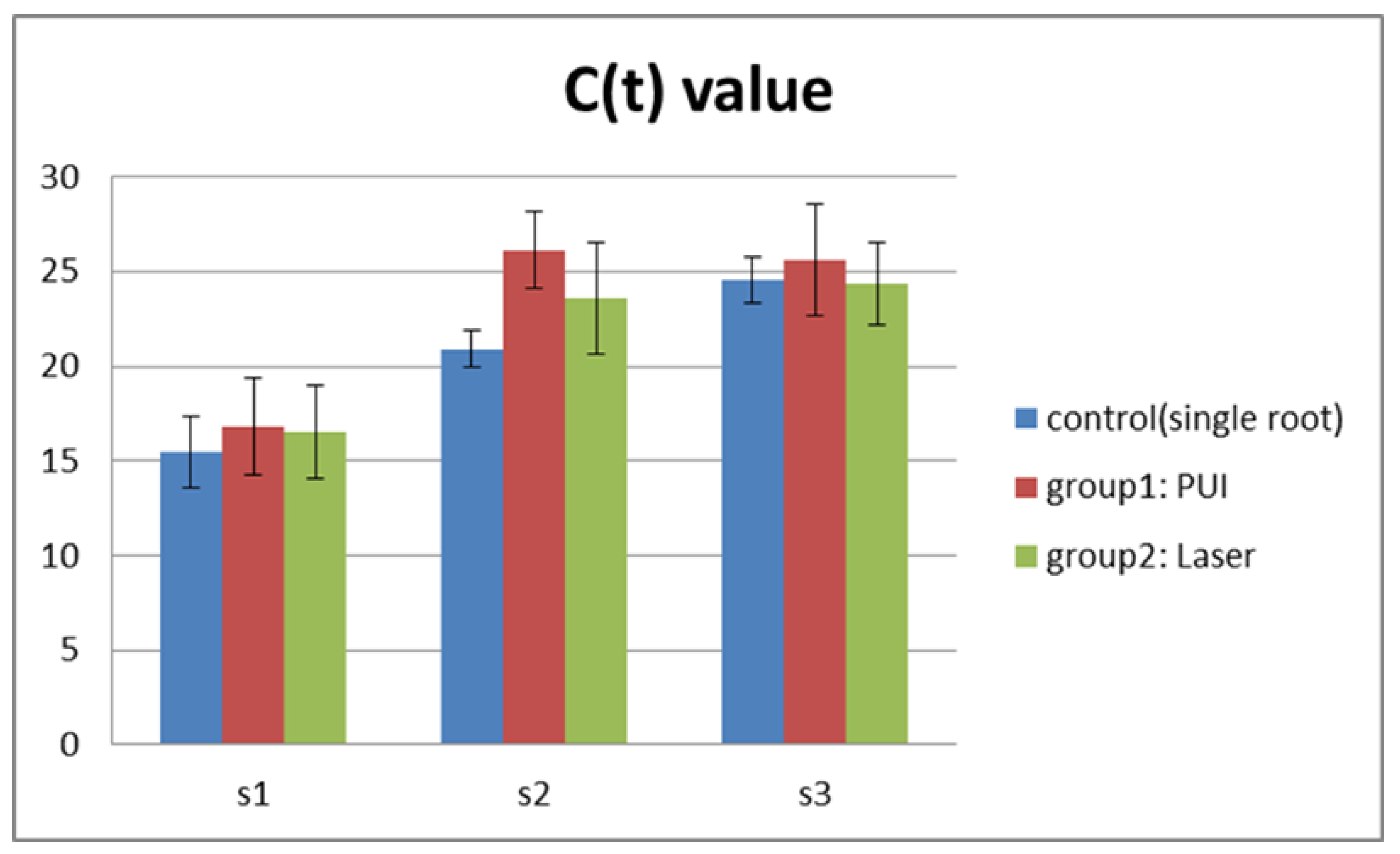
3.1. Microbiological Analysis
3.2. SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nair, P.N.R.; Henry, S.; Cano, V.; Vera, J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef]
- Harrison, A.J.; Chivatxaranukul, P.; Parashos, P.; Messer, H.H. The effect of ultrasonically activated irrigation on reduction of Enterococcus faecalis in experimentally infected root canals. Int. Endod. J. 2010, 43, 968–977. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Clinical implications and microbiology of bacterial persistence after treatment procedures. J. Endod. 2008, 34, 1291–1301. [Google Scholar] [CrossRef]
- Peters, O.A. Current challenges and concepts in the preparation of root canal systems: A review. J. Endod. 2004, 30, 559–567. [Google Scholar] [CrossRef]
- Weller, R.N.; Brady, J.M.; Bernier, W.E. Efficacy of ultrasonic cleaning. J. Endod. 1980, 6, 740–743. [Google Scholar] [CrossRef]
- Orstavik, D.; Haapasalo, M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod. Dent. Traumatol. 1990, 6, 142–149. [Google Scholar] [CrossRef]
- Gründling, G.L.; Zechin, J.G.; Jardim, W.M.; de Oliveira, S.D.; de Figueiredo, J.A.P. Effect of ultrasonics on Enterococcus faecalis biofilm in a bovine tooth model. J. Endod. 2011, 37, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Shigetani, Y.; Sasa, N.; Suzuki, H.; Okiji, T.; Ohshima, H. GaAlAs laser irradiation induces active tertiary dentin formation after pulpal apoptosis and cell proliferation in rat molars. J. Endod. 2011, 37, 1086–1091. [Google Scholar] [CrossRef]
- Yazdanfar, I.; Gutknecht, N.; Franzen, R. Effects of diode laser on direct pulp capping treatment: A pilot study. Lasers Med. Sci. 2015, 30, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Silva Garcez, A.; Núñez, S.C.; Lage-Marques, J.L.; Jorge, A.O.C.; Ribeiro, M.S. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e93–e98. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.B.; Cai, S.; Simionato, M.R.L.; Lage-Marques, J.L. High-power diode laser in the disinfection in depth of the root canal dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, e68–e72. [Google Scholar] [CrossRef]
- Azim, A.A.; Aksel, H.; Zhuang, T.; Mashtare, T.; Babu, J.P.; Huang, G.T.-J. Efficacy of 4 Irrigation Protocols in Killing Bacteria Colonized in Dentinal Tubules Examined by a Novel Confocal Laser Scanning Microscope Analysis. J. Endod. 2016, 42, 928–934. [Google Scholar] [CrossRef]
- Căpută, P.E.; Retsas, A.; Kuijk, L.; Chávez de Paz, L.E.; Boutsioukis, C. Ultrasonic Irrigant Activation during Root Canal Treatment: A Systematic Review. J. Endod. 2019, 45, 31–44. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-activated Nanoparticles: An in Vitro Study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, N.; van Gogswaardt, D.; Conrads, G.; Apel, C.; Schubert, C.; Lampert, F. Diode laser radiation and its bactericidal effect in root canal wall dentin. J. Clin. Laser Med. Surg. 2000, 18, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Card, S.J.; Sigurdsson, A.; Orstavik, D.; Trope, M. The effectiveness of increased apical enlargement in reducing intracanal bacteria. J. Endod. 2002, 28, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Cheng, C.Q.; Mohanraj, R.; Sriraman, P.; Subbarao, C.; Sharma, S. Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er:YAG laser in vitro. Int. Endod. J. 2015, 48, 602–610. [Google Scholar] [CrossRef]
- Meire, M.A.; Coenye, T.; Nelis, H.J.; De Moor, R.J.G. Evaluation of Nd:YAG and Er:YAG irradiation, antibacterial photodynamic therapy and sodium hypochlorite treatment on Enterococcus faecalis biofilms. Int. Endod. J. 2012, 45, 482–491. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Wyrsch, H.; Bürklein, S.; Schäfer, E. Removal of Calcium Hydroxide from Artificial Grooves in Straight Root Canals: Sonic Activation Using EDDY Versus Passive Ultrasonic Irrigation and XPendo Finisher. J. Endod. 2019, 45, 322–326. [Google Scholar] [CrossRef] [PubMed]
- da Costa Ribeiro, A.; Nogueira, G.E.C.; Antoniazzi, J.H.; Moritz, A.; Zezell, D.M. Effects of diode laser (810 nm) irradiation on root canal walls: Thermographic and morphological studies. J. Endod. 2007, 33, 252–255. [Google Scholar] [CrossRef]
- Passalidou, S.; Calberson, F.; De Bruyne, M.; De Moor, R.; Meire, M.A. Debris Removal from the Mesial Root Canal System of Mandibular Molars with Laser-activated Irrigation. J. Endod. 2018, 44, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- da Costa Lima, G.A.; Aguiar, C.M.; Câmara, A.C.; Alves, L.C.; Dos Santos, F.A.B.; do Nascimento, A.E. Comparison of smear layer removal using the Nd:YAG laser, ultrasound, ProTaper Universal system, and CanalBrush methods: An in vitro study. J. Endod. 2015, 41, 400–404. [Google Scholar] [CrossRef] [PubMed]
- De Moor, R.J.G.; Meire, M.; Goharkhay, K.; Moritz, A.; Vanobbergen, J. Efficacy of ultrasonic versus laser-activated irrigation to remove artificially placed dentin debris plugs. J. Endod. 2010, 36, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Jurič, I.B.; Anić, I. The Use of Lasers in Disinfection and Cleanliness of Root Canals: A Review. Acta Stomatol. Croat. 2014, 48, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Golob, B.S.; Olivi, G.; Vrabec, M.; El Feghali, R.; Parker, S.; Benedicenti, S. Efficacy of Photon-induced Photoacoustic Streaming in the Reduction of Enterococcus faecalis within the Root Canal: Different Settings and Different Sodium Hypochlorite Concentrations. J. Endod. 2017, 43, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Sasanakul, P.; Ampornaramveth, R.S.; Chivatxaranukul, P. Influence of Adjuncts to Irrigation in the Disinfection of Large Root Canals. J. Endod. 2019, 45, 332–337. [Google Scholar] [CrossRef]
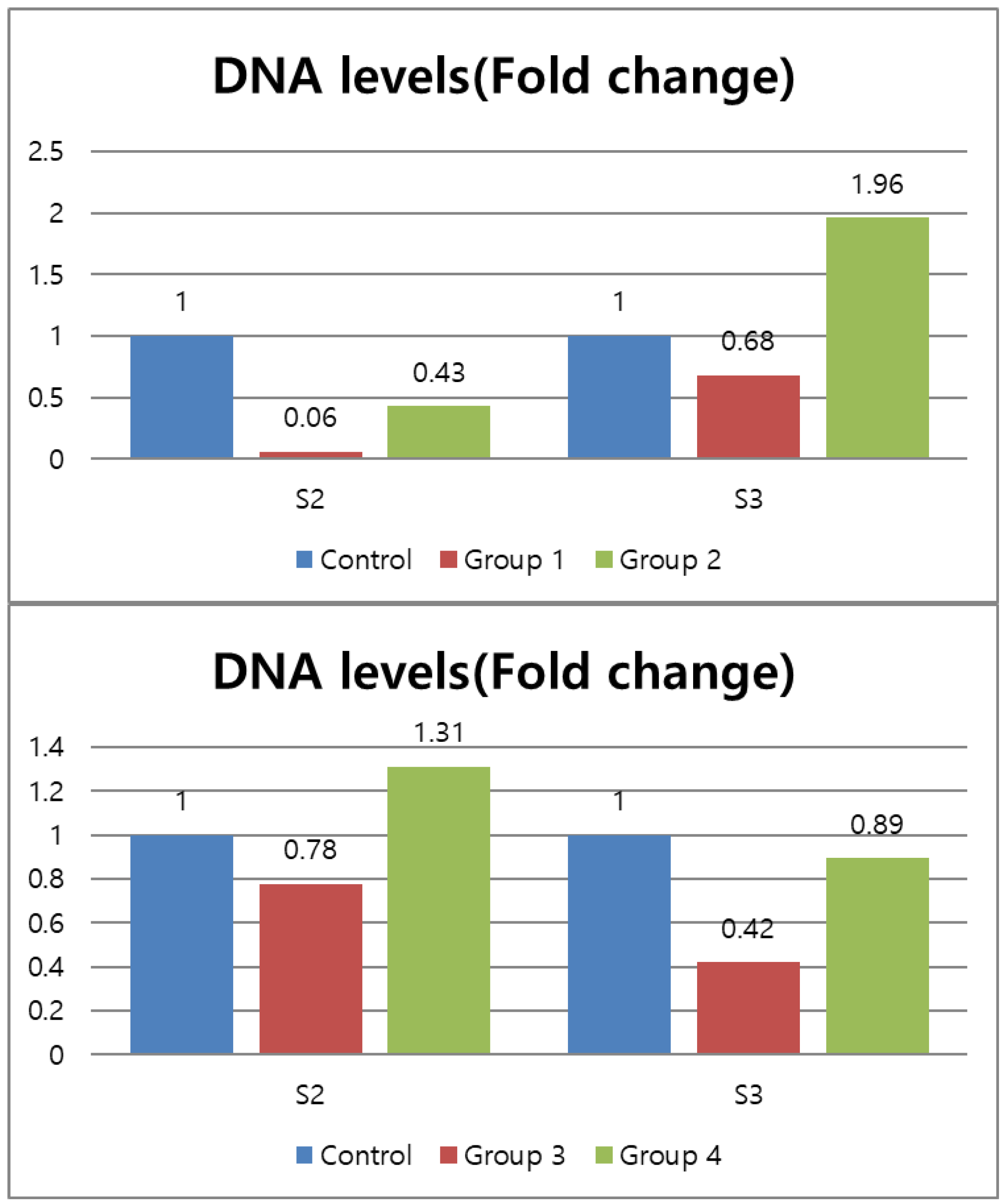

| Subgroup | S1 | S2 | S3 | p-Value |
|---|---|---|---|---|
| Group1 (n = 20) | 0.8927 ± 3.30 z | 0.001 ± 0.00 cy | 0.001 ± 0.02 y | <0.001 |
| Group2 (n = 20) | 0.8812 ± 1.95 z | 0.006 ± 0.01 by | 0.002 ± 0.00 y | <0.001 |
| Control (n = 3, single rooted) | 1.0 ± 1.72 z | 0.013 ± 0.01 ay | 0.001 ± 0.00 x | <0.001 |
| p-value | 0.99 | <0.001 | 0.07 | - |
| Group3 (n = 20) | 1.0770 ± 4.52 z | 0.003 ± 0.01 y | 0.001 ± 0.00 bx | <0.001 |
| Group4 (n = 20) | 0.8985 ± 5.10 z | 0.006 ± 0.10 y | 0.002 ± 0.00 ax | <0.001 |
| Control (n = 3, double rooted) | 1 ± 1.91 z | 0.005 ± 0.00 y | 0.002 ± 0.00 aby | <0.001 |
| p-value | 0.98 | 0.504 | <0.05 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-E.; Kim, Y.-M. Comparison of Anti-Bacterial Efficacy between Passive Ultrasonic Irrigation and 980 nm-GaAlAs Laser Application in Two Root Types. Medicina 2021, 57, 537. https://doi.org/10.3390/medicina57060537
Yang S-E, Kim Y-M. Comparison of Anti-Bacterial Efficacy between Passive Ultrasonic Irrigation and 980 nm-GaAlAs Laser Application in Two Root Types. Medicina. 2021; 57(6):537. https://doi.org/10.3390/medicina57060537
Chicago/Turabian StyleYang, Sung-Eun, and Yong-Min Kim. 2021. "Comparison of Anti-Bacterial Efficacy between Passive Ultrasonic Irrigation and 980 nm-GaAlAs Laser Application in Two Root Types" Medicina 57, no. 6: 537. https://doi.org/10.3390/medicina57060537
APA StyleYang, S.-E., & Kim, Y.-M. (2021). Comparison of Anti-Bacterial Efficacy between Passive Ultrasonic Irrigation and 980 nm-GaAlAs Laser Application in Two Root Types. Medicina, 57(6), 537. https://doi.org/10.3390/medicina57060537






