Vitamin D and VDR Gene Polymorphisms’ Association with Rheumatoid Arthritis in Lithuanian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Vitamin D Evaluation
2.3. Cell Preparation and DNA Isolation
2.4. Quantitative Polymerase Chain Reaction (qPCR) and VDR Genotyping
2.5. Statistical Analysis
3. Results
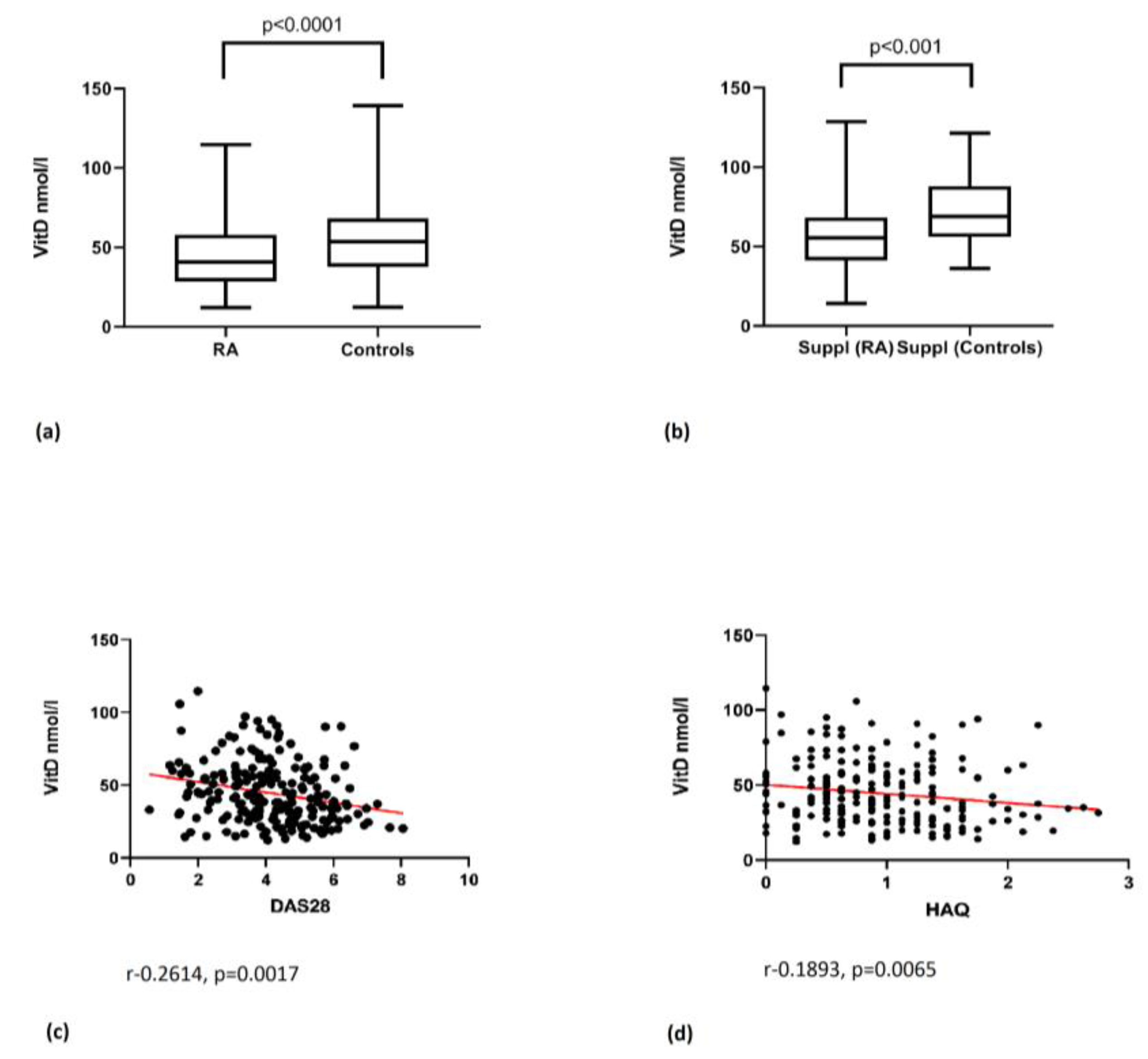
3.1. Vitamin D Level and Its Correlation with Disease Activity Parameters
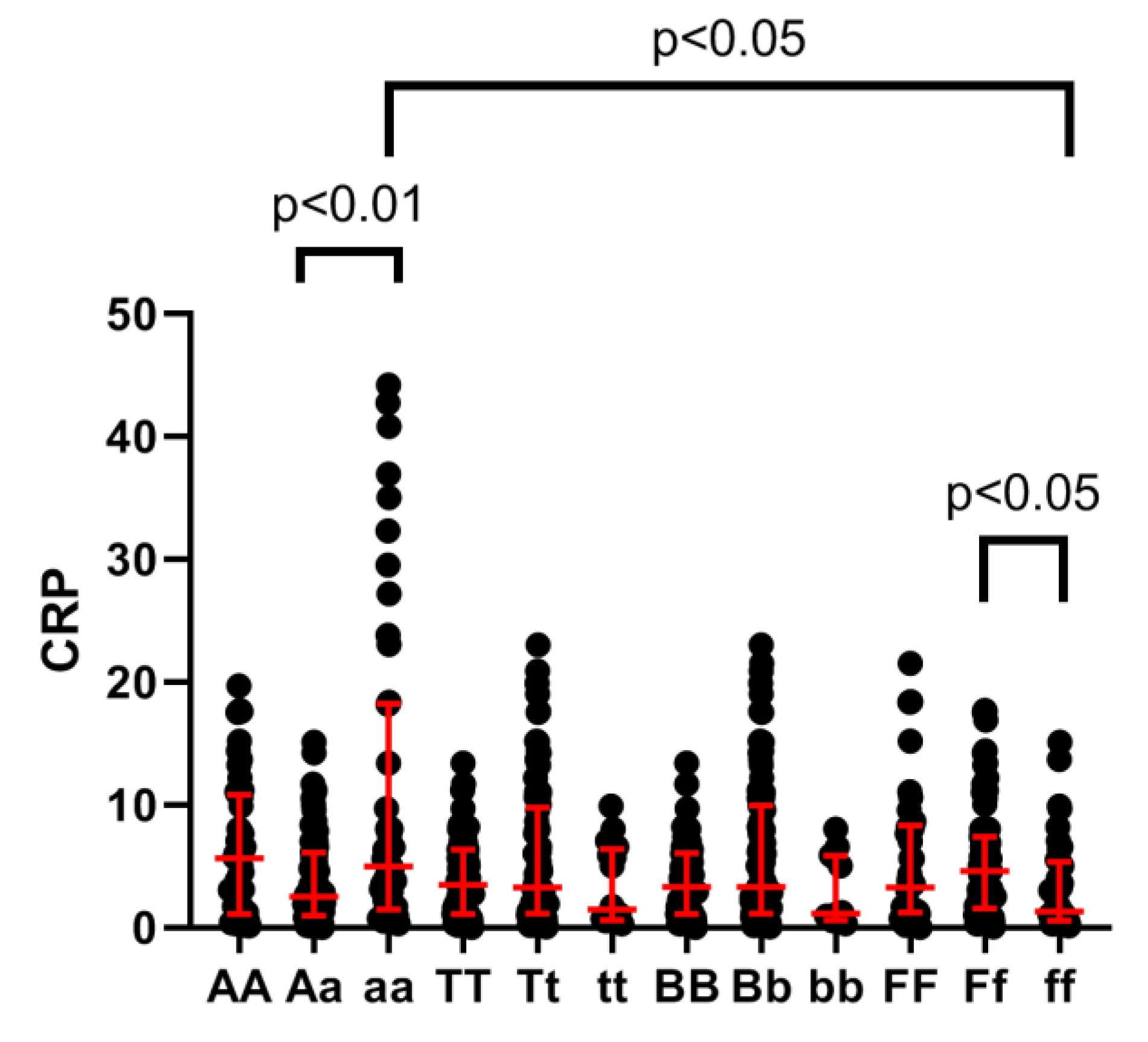
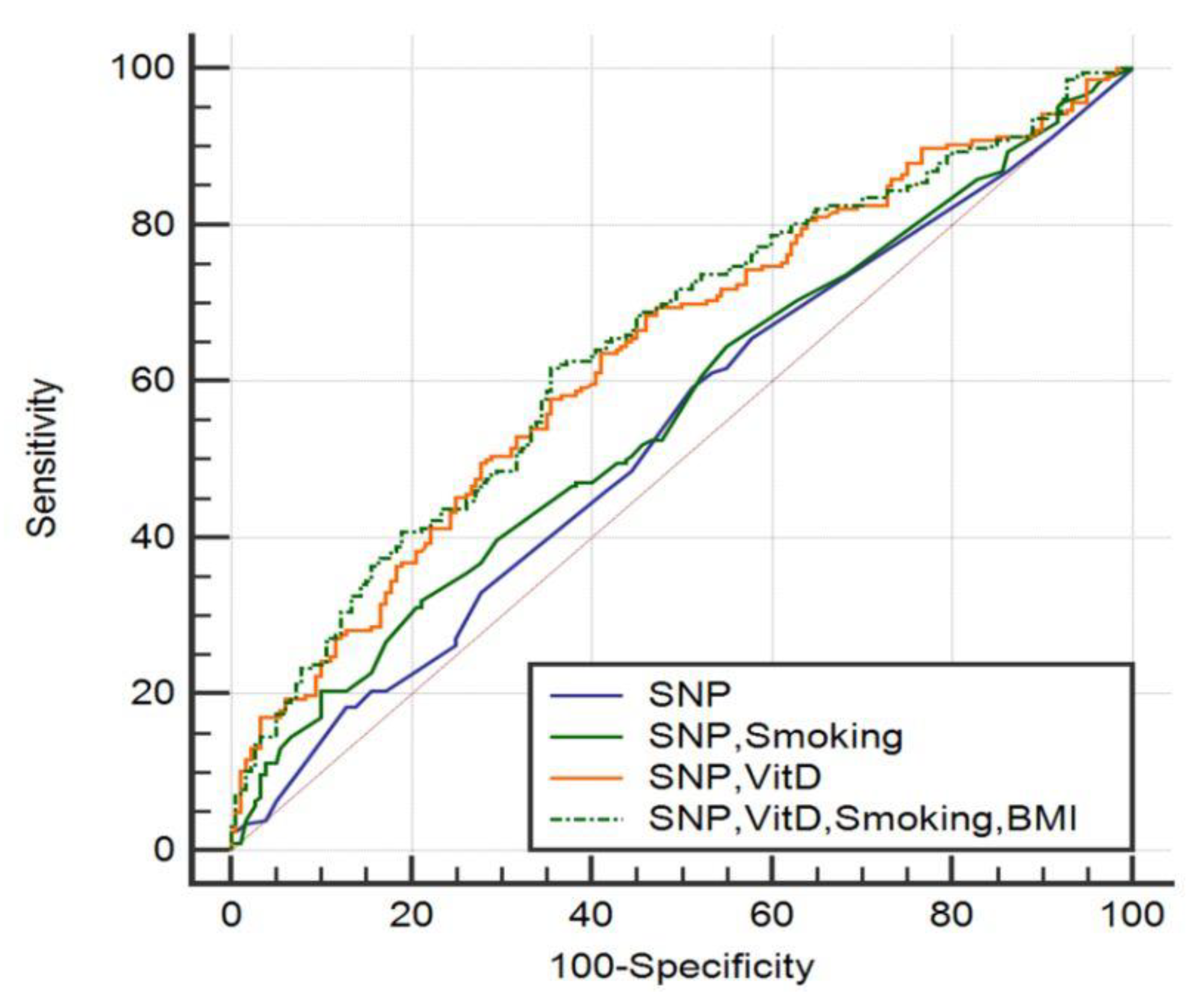
3.2. VDR Polymorphism Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, E.; Petrelli, F.; Bonifacio, A.F.; Puxeddu, I.; Alunno, A. One year in review 2018: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 175–184. [Google Scholar] [PubMed]
- Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D in rheumatoid arthritis-towards clinical application. Nat. Rev. Rheumatol. 2016, 12, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of Vitamin D Beyond the Skeletal Function: A Review of the Molecular and Clinical Studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [PubMed]
- Orbach, H.; Zandman-Goddard, G.; Amital, H.; Barak, V.; Szekanecz, Z.; Szucs, G.; Danko, K.; Nagy, E.; Csepany, T.; Carvalho, J.F.; et al. Novel biomarkers in autoimmune diseases: Prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann. N. Y. Acad Sci. 2007, 1109, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Watad, A.; Neumann, S.G.; Simon, M.; Brown, S.B.; Abu Much, A.; Harari, A.; Tiosano, S.; Amital, H.; Shoenfeld, Y. Vitamin D and rheumatoid arthritis: An ongoing mystery. Curr. Opin. Rheumatol. 2017, 29, 378–388. [Google Scholar] [CrossRef]
- Welsh, P.; Peters, M.J.; McInnes, I.B.; Lems, W.F.; Lips, P.T.; McKellar, G.; Knox, S.; Michael Wallace, A.; Dijkmans, B.A.; Nurmohamed, M.T.; et al. Vitamin D deficiency is common in patients with RA and linked to disease activity, but circulating levels are unaffected by TNFα blockade: Results from a prospective cohort study. Ann. Rheum. Dis. 2011, 70, 1165–1167. [Google Scholar] [CrossRef]
- Cutolo, M.; Otsa, K.; Laas, K.; Yprus, M.; Lehtme, R.; Secchi, M.E.; Sulli, A.; Paolino, S.; Seriolo, B. Circannual vitamin D serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin. Exp. Rheumatol. 2006, 24, 702–704. [Google Scholar]
- Agmon-Levin, N.; Theodor, E.; Segal, R.M.; Shoenfeld, Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin. Rev. Allergy Immunol. 2013, 45, 256–266. [Google Scholar] [CrossRef]
- Uitterlinden, A.G.; Fang, Y.; van Meurs, J.B.; van Leeuwen, H.; Pols, H.A. Vitamin D receptor gene polymorphisms in relation to Vitamin D related disease states. J. Steroid. Biochem. Mol. Biol. 2004, 89-90, 187–193. [Google Scholar] [CrossRef]
- Ranganathan, P. Genetics of bone loss in rheumatoid arthritis—role of vitamin D receptor polymorphisms. Rheumatology 2009, 48, 342–346. [Google Scholar] [CrossRef]
- Rukin, N.J.; Strange, R.C. What are the frequency, distribution, and functional effects of vitamin D receptor polymorphisms as related to cancer risk? Nutr. Rev. 2007, 65, S96–S101. [Google Scholar] [CrossRef]
- Song, G.G.; Bae, S.C.; Lee, Y.H. Vitamin D receptor FokI, BsmI, and TaqI polymorphisms and susceptibility to rheumatoid arthritis. Z. Rheumatol. 2016, 75, 322–329. [Google Scholar] [CrossRef]
- Saccone, D.; Asani, F.; Bornman, L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene 2015, 561, 171–180. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Bruce, B.; Fries, J.F. The health assessment questionnaire (HAQ). Clin. Exp. Rheumatol. 2005, 23 (Suppl. 39), S14–S18. [Google Scholar] [PubMed]
- Gossec, L.; Dougados, M.; Rincheval, N.; Balanescu, A.; Boumpas, D.T.; Canadelo, S.; Carmona, L.; Daurès, J.P.; de Wit, M.; Dijkmans, B.A.; et al. Elaboration of the preliminary Rheumatoid Arthritis Impact of Disease (RAID) score: A EULAR initiative. Ann. Rheum. Dis. 2009, 68, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Laragione, T.; Shah, A.; Gulko, P.S. The vitamin D receptor regulates rheumatoid arthritis synovial fibroblast invasion and morphology. Mol. Med. 2012, 18, 194–200. [Google Scholar] [CrossRef]
- Fan, P.; He, L.; Hu, N.; Luo, J.; Zhang, J.; Mo, L.F.; Wang, Y.H.; Pu, D.; Lv, X.H.; Hao, Z.M.; et al. Effect of 1, 25-(OH) 2D3 on proliferation of fibroblast-like synoviocytes and expressions of pro-inflammatory cytokines through regulating MicroRNA-22 in a rat model of rheumatoid arthritis. Cell Physiol. Biochem. 2017, 42, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Zwerina, K.; Baum, W.; Axmann, R.; Heiland, G.R.; Distler, J.H.; Smolen, J.; Hayer, S.; Zwerina, J.; Schett, G. Vitamin D receptor regulates TNF-mediated arthritis. Ann. Rheum. Dis. 2011, 70, 1122–1129. [Google Scholar] [CrossRef]
- Song, G.G.; Bae, S.C.; Lee, Y.H. Association between vitamin D intake and the risk of rheumatoid arthritis: A meta-analysis. Clin. Rheumatol. 2012, 31, 1733–1739. [Google Scholar] [CrossRef]
- Hajjaj-Hassouni, N.; Mawani, N.; Allali, F.; Rkain, H.; Hassouni, K.; Hmamouchi, I.; Dougados, M. Evaluation of vitamin D status in rheumatoid arthritis and its association with disease activity across 15 countries: “The COMORA Study”. Int. J. Rheumatol. 2017. [Google Scholar] [CrossRef]
- Vojinovic, J.; Tincani, A.; Sulli, A.; Soldano, S.; Andreoli, L.; Dall’Ara, F.; Ionescu, R.; Pasalic, K.S.; Balcune, I.; Ferraz-Amaro, I.; et al. European multicentre pilot survey to assess vitamin D status in rheumatoid arthritis patients and early development of a new Patient Reported Outcome questionnaire (D-PRO). Autoimmun. Rev. 2017, 16, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A meta-analysis. Clin. Exp. Rheumatol. 2016, 34, 827–833. [Google Scholar] [PubMed]
- Lin, J.; Liu, J.; Davies, M.L.; Chen, W. Serum vitamin D level and rheumatoid arthritis disease activity: Review and meta-analysis. PLoS ONE 2016, 11, e0146351. [Google Scholar] [CrossRef]
- Raczkiewicz, A.; Kisiel, B.; Kulig, M.; Tłustochowicz, W. Vitamin D status and its association with quality of life, physical activity, and disease activity in rheumatoid arthritis patients. J. Clin. Rheumatol. 2015, 21, 126–130. [Google Scholar] [CrossRef]
- Chandrashekara, S.; Patted, A. Role of vitamin D supplementation in improving disease activity in rheumatoid arthritis: An exploratory study. Int. J. Rheum. Dis. 2017, 20, 825–831. [Google Scholar] [CrossRef]
- Polasik, K.; Piotrowska, E.; Lipińska, B.; Witkowski, J.M.; Bryl, E.; Tukaj, S. Vitamin D status in patients with rheumatoid arthritis: Correlation analysis with disease activity and progression, as well as serum IL-6 levels. Acta Biochim. Pol. 2017, 64, 667–670. [Google Scholar] [CrossRef]
- Higgins, M.J.; Mackie, S.L.; Thalayasingam, N.; Bingham, S.J.; Hamilton, J.; Kelly, C.A. The effect of vitamin D levels on the assessment of disease activity in rheumatoid arthritis. Clin. Rheumatol. 2013, 32, 863–867. [Google Scholar] [CrossRef]
- Di Spigna, G.; Del Puente, A.; Covelli, B.; Abete, E.; Varriale, E.; Salzano, S.; Postiglione, L. Vitamin D receptor polymorphisms as tool for early screening of severe bone loss in women patients with rheumatoid arthritis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4664–4669. [Google Scholar] [PubMed]
- Garcia-Lozano, J.R.; Gonzalez-Escribano, M.F.; Valenzuela, A.; Garcia, A.; Núñez-Roldán, A. Association of vitamin D receptor genotypes with early onset rheumatoid arthritis. Eur. J. Immunogenet. 2001, 28, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Goertz, B.; Fassbender, W.J.; Williams, J.C.; Marzeion, A.M.; Bretzel, R.G.; Stracke, H.; Berliner, M.N. Vitamin D receptor genotypes are not associated with rheumatoid arthritis or biochemical parameters of bone turnover in German RA patients. Clin. Exp. Rheumatol. 2003, 21, 333–339. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C.; Choi, S.J.; Ji, J.D.; Song, G.G. Associations between vitamin D receptor polymorphisms and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: A meta-analysis. Mol. Biol. Rep. 2011, 38, 3643–3651. [Google Scholar] [CrossRef]
- Maalej, A.; Petit-Teixeira, E.; Michou, L.; Rebai, A.; Cornelis, F.; Ayadi, H. Association study of VDR gene with rheumatoid arthritis in the French population. Genes Immun. 2005, 6, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Mayne, C.G.; Spanier, J.A.; Relland, L.M.; Williams, C.B.; Hayes, C.E. 1, 25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2011, 41, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; Alperi-López, M.; Naves-Díaz, M.; Dusso, A.; López, P.; Ballina-García, F.J.; Cannata-Andía, J.B.; Suárez, A. Vitamin D Receptor Polymorphism and DHCR7 Contribute to the Abnormal Interplay Between Vitamin D and Lipid Profile in Rheumatoid Arthritis. Sci. Rep. 2019, 9, 2546. [Google Scholar] [CrossRef]
- Monticielo, O.A.; Brenol, J.C.; Chies, J.A.; Longo, M.G.; Rucatti, G.G.; Scalco, R.; Xavier, R.M. The role of Bsm I and Fok I vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D in Brazilian patients with systemic lupus erythematosus. Lupus 2012, 21, 43–52. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.; Verlinden, L.; Giulietti, A.; Ramos-Lopez, E.; Branisteanu, D.D.; Ferreira, G.B.; Overbergh, L.; Verstuyf, A.; Bouillon, R.; Roep, B.O.; et al. The vitamin D receptor gene FokIpolymorphism: Functional impact on the immune system. Eur. J. Immunol. 2007, 37, 395–405. [Google Scholar] [CrossRef] [PubMed]
| Parameters | RA (n = 206) | Controls (n = 180) | p Significance |
|---|---|---|---|
| Gender (Male/Female) | 22/184 | 17/163 | 0.6879 |
| Age | 55.01 ± 11.08 | 53.15 ± 10.68 | 0.0975 |
| BMI (kg/m2) | 25.62 ± 4.64 | 26.46 ± 4.74 | 0.1005 |
| 25(OH)D (nmol/L): | 44.96 ± 21.92 | 54.90 ± 22.82 | p < 0.0001 * |
| Deficiency | 127 (61.65) | 79 (43.89) | |
| Insufficiency | 57 (27.67) | 73 (40.56) | |
| Normal range | 20 (9.7) | 28 (15.56) | |
| Smoking (n): | 0.0619 | ||
| Yes | 34 | 18 | |
| No | 172 | 162 | |
| Disease onset (Years) | 43.30 ± 12.80 | - | |
| RA duration (Years) | 11.71 ± 9.22 | - | |
| DAS28 CRP: | 4.16 ± 1.46 | - | |
| High activity | 56 (27.18) | ||
| Moderate activity | 98 (47.57) | ||
| Low activity | 19 (9.22) | ||
| Remission | 33 (16.01) | ||
| HAQ | 0.95 ± 0.61 | - | |
| RAID | 4.74 ± 2.23 | - | |
| CRP (mg/L) | 12.55 ± 21.7 | - | |
| VAS (mm) | 45.57 ± 20.88 | - |
| Genotype Frequency n (%) | Allele Frequency n (%) | OR (95% CI) | HWE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ApaI | AA | Aa | aa | X2(p) | A | a | X2(p) | X2(p) | |
| RA (n = 206) | 56 (27) | 99 (48) | 51 (25) | 1.172 | 211 (51) | 201 (49) | 0.322 | 0.983 (0.618–1.564) | 0.302 (0.583) |
| Controls (n = 180) | 41 (23) | 95 (53) | 44 (24) | (0.557) | 177 (49) | 183 (51) | (0.570) | 1.085 (0.818– 1.440) | 0.561 (0.454) |
| TaqI | TT | Tt | tt | T | t | ||||
| RA (206) | 95 (46) | 92 (45) | 19 (9) | 0.014 | 282 (68) | 130 (32) | 0.002 | 1.041 (0.519–2.092) | 0.237 (0.626) |
| Controls (180) | 83 (46) | 81 (45) | 16 (9) | (0.993) | 247 (69) | 113 (31) | (0.961) | 1.008 (0.743–1.366) | 0.360 (0.548) |
| BsmI | BB | Bb | bb | B | b | ||||
| RA (206) | 91 (44) | 101 (49) | 14 (7) | 0.036 | 283 (69) | 129 (31) | 0.001 | 0.937 (0.428– 2.049) | 4.028 (0.045) |
| Controls (180) | 80 (45) | 87 (48) | 13 (7) | (0.982) | 247 (69) | 113 (31) | (0.981) | 0.996 (0.735– 1.351) | 2.685 (0.101) |
| FokI | FF | Ff | ff | F | f | ||||
| RA (206) | 43 (21) | 110 (53) | 53 (26) | 0.085 | 196 (48) | 216 (52) | 0.045 | 1.071 (0.675– 1.699) | 1.023 (0.312) |
| Controls (180) | 38 (21) | 98 (55) | 44 (24) | (0.958) | 174 (48) | 186 (52) | (0.832) | 1.031 (0.777– 1.368) | 1.461 (0.227) |
| Genotype | RA | Controls | p Value |
|---|---|---|---|
| Vitamin D Level (nmol/L, M ± SD) | |||
| ApaI | |||
| AA | 43.46 ± 23.52 | 54.89 ± 21.7 | 0.0034 * |
| Aa | 45.3 ±22.37 | 53.71 ± 23.05 | 0.009 * |
| aa | 45.87 ± 19.44 | 57.49 ± 23.64 | 0.0087 * |
| TaqI | |||
| TT | 46.2 ± 20.65 | 56.47 ± 24.3 | 0.0014 * |
| Tt | 42.86 ± 24.29 | 53.11 ± 21.33 | 0.0003 * |
| tt | 48.88 ± 14.8 | 55.86 ± 23.02 | 0.2869 |
| BsmI | |||
| BB | 46.62 ± 20.71 | 56.78 ± 23.66 | 0.0012 * |
| Bb | 43.15 ± 23.97 | 53.62 ± 22.16 | 0.0002 * |
| bb | 47.16 ± 11.9 | 51.89 ± 22.83 | 0.8952 |
| FokI | |||
| FF | 42.54 ± 18.62 | 54.99 ± 24.1 | 0.0123 * |
| Ff | 44.97 ± 23.75 | 56.12 ± 22.08 | <0.0001 * |
| ff | 43.4 ± 20.59 | 46.0 ± 23.62 | 0.2143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punceviciene, E.; Gaizevska, J.; Sabaliauskaite, R.; Venceviciene, L.; Puriene, A.; Vitkus, D.; Jarmalaite, S.; Butrimiene, I. Vitamin D and VDR Gene Polymorphisms’ Association with Rheumatoid Arthritis in Lithuanian Population. Medicina 2021, 57, 346. https://doi.org/10.3390/medicina57040346
Punceviciene E, Gaizevska J, Sabaliauskaite R, Venceviciene L, Puriene A, Vitkus D, Jarmalaite S, Butrimiene I. Vitamin D and VDR Gene Polymorphisms’ Association with Rheumatoid Arthritis in Lithuanian Population. Medicina. 2021; 57(4):346. https://doi.org/10.3390/medicina57040346
Chicago/Turabian StylePunceviciene, Egle, Justina Gaizevska, Rasa Sabaliauskaite, Lina Venceviciene, Alina Puriene, Dalius Vitkus, Sonata Jarmalaite, and Irena Butrimiene. 2021. "Vitamin D and VDR Gene Polymorphisms’ Association with Rheumatoid Arthritis in Lithuanian Population" Medicina 57, no. 4: 346. https://doi.org/10.3390/medicina57040346
APA StylePunceviciene, E., Gaizevska, J., Sabaliauskaite, R., Venceviciene, L., Puriene, A., Vitkus, D., Jarmalaite, S., & Butrimiene, I. (2021). Vitamin D and VDR Gene Polymorphisms’ Association with Rheumatoid Arthritis in Lithuanian Population. Medicina, 57(4), 346. https://doi.org/10.3390/medicina57040346





