A Pilot Study of Safer Radiation Dosage to the Heart and Its Subregions
Abstract
1. Introduction
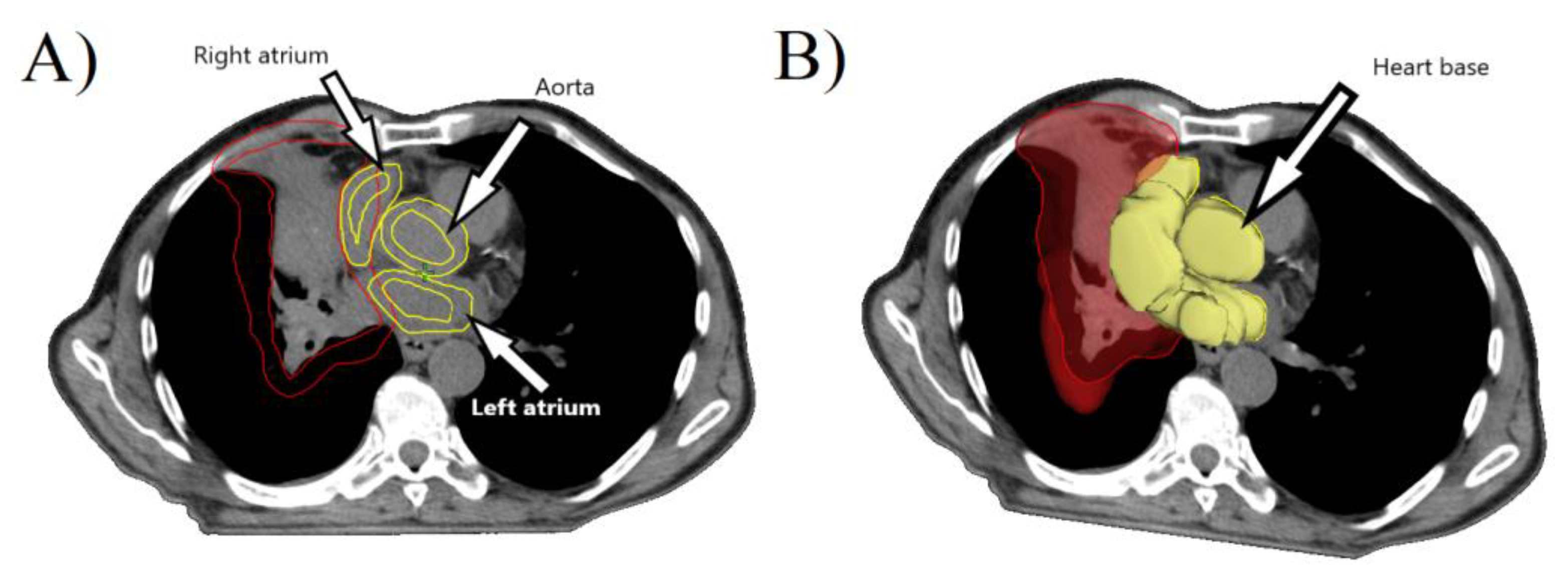
2. Materials and Methods
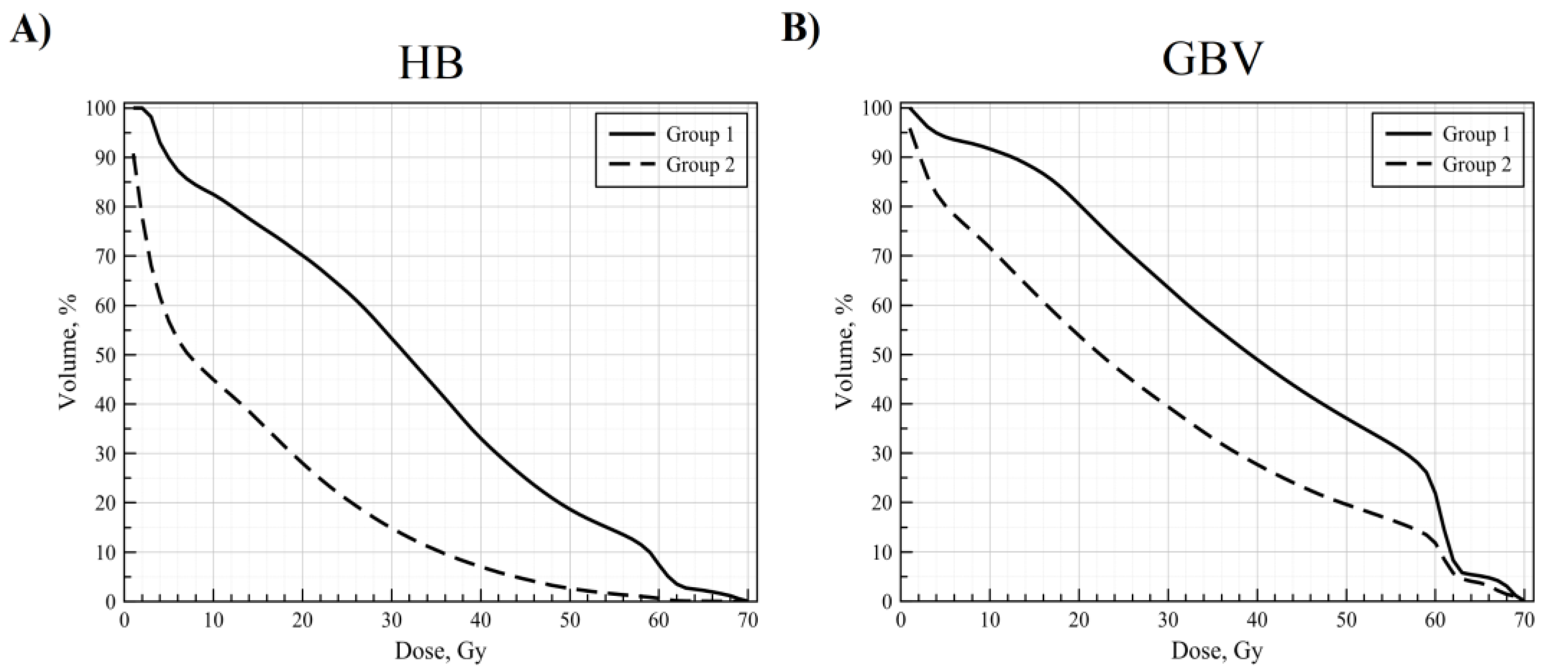
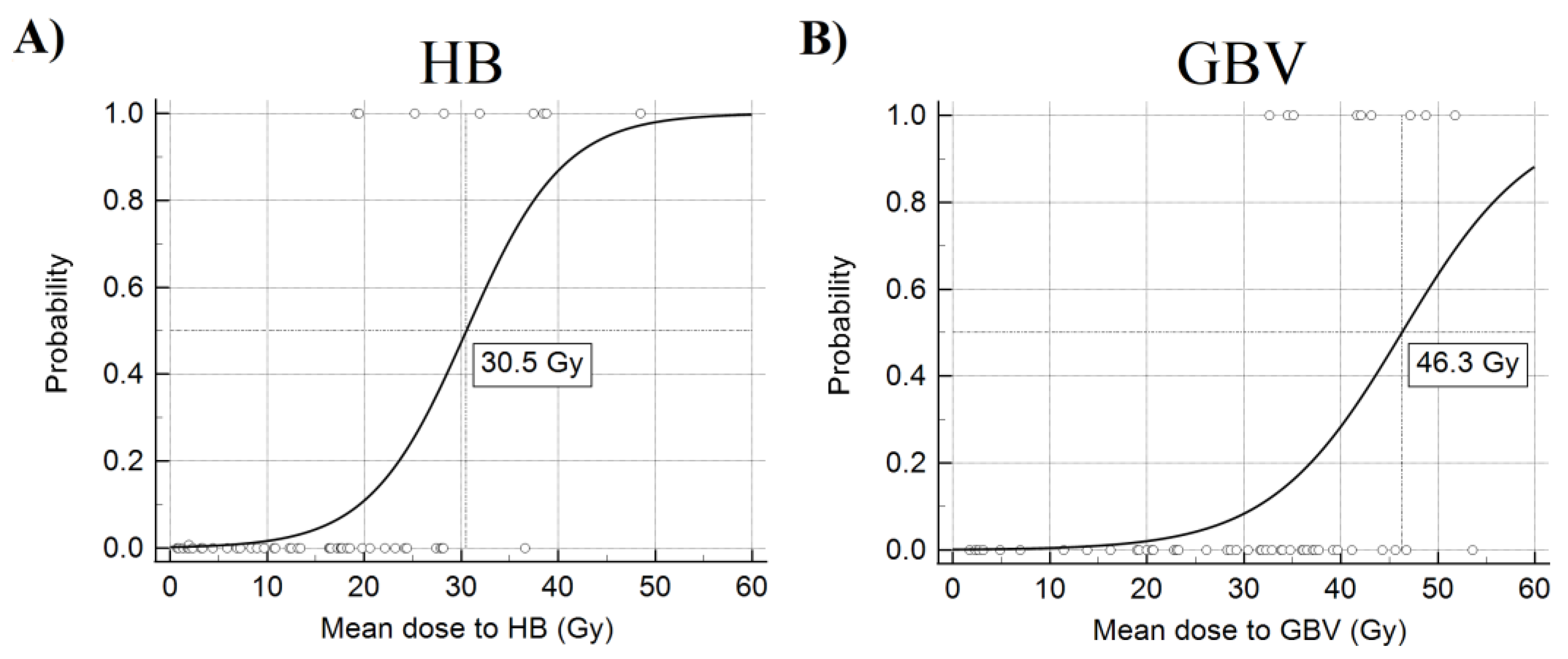
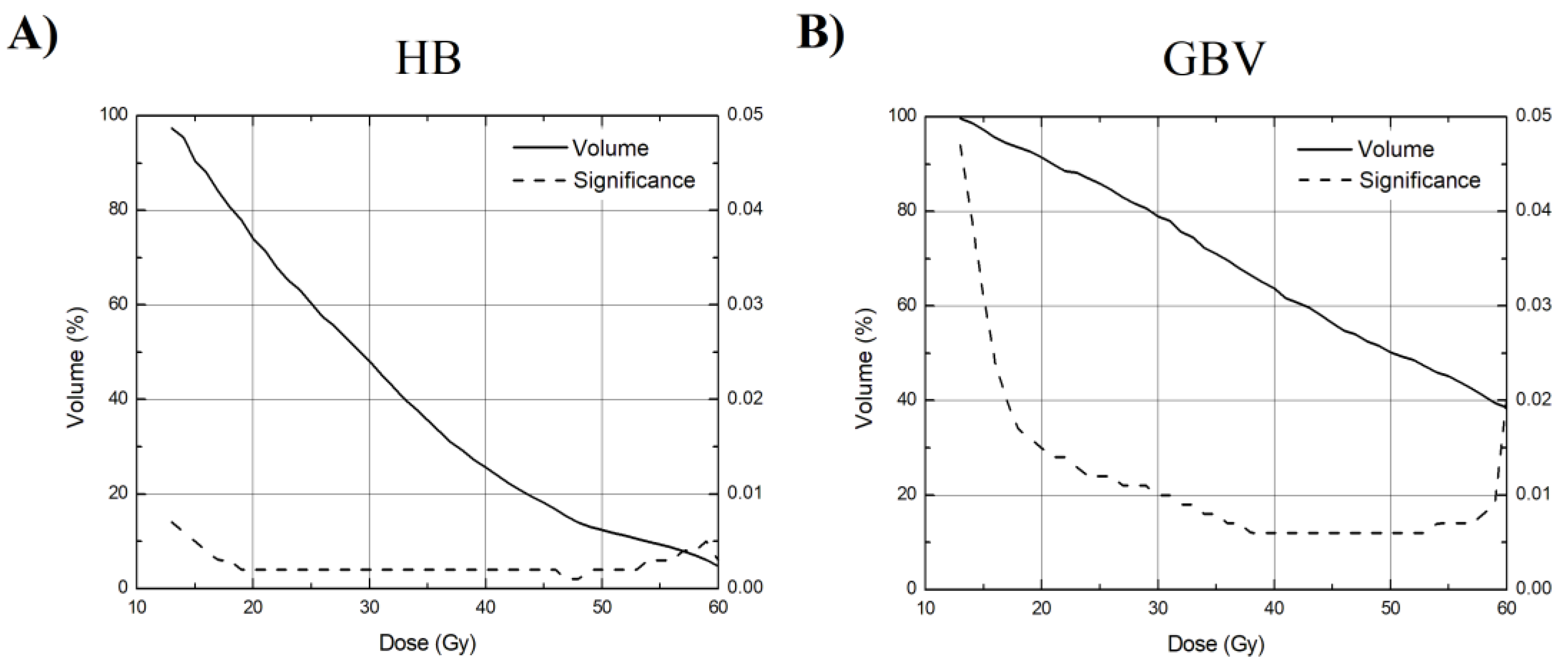
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Majem, M.; Hernįndez-Hernįndez, J.; Hernando-Trancho, F.; Rodrķguez de Dios, N.; Sotoca, A.; Trujillo-Reyes, J.C.; Vollmer, I.; Delgado-Bolton, R.; Provencio, M. Multidis-ciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clin. Transl. Oncol. 2020, 22, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Schytte, T.; Hansen, O.; Stolberg-Rohr, T.; Brink, C. Cardiac toxicity and radiation dose to the heart in definitive treated non-small cell lung cancer. Acta Oncol. 2010, 49, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Veinot, J.P.; Edwards, W.D. Pathology of radiation-induced heart disease: A surgical and autopsy study of 27 cases. Hum. Pathol. 1996, 27, 766–773. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal ra-diotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [PubMed]
- Bradley, J.D.; Hu, C.; Komaki, R.R.; Masters, G.A.; Blumenschein, G.R.; Schild, S.E.; Bogart, J.A.; Forster, K.M.; Magliocco, A.M.; Kavadi, V.S.; et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 706–714. [Google Scholar] [CrossRef]
- Atkins, K.M.; Rawal, B.; Chaunzwa, T.L.; Lamba, N.; Bitterman, D.S.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Chen, A.B.; Nguyen, P.L.; et al. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J. Am. Coll. Cardiol. 2019, 73, 2976–2987. [Google Scholar] [CrossRef]
- McWilliam, A.; Kennedy, J.; Hodgson, C.; Osorio, E.V.; Faivre-Finn, C.; Van Herk, M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur. J. Cancer 2017, 85, 106–113. [Google Scholar] [CrossRef]
- Jang, B.-S.; Cha, M.-J.; Kim, H.J.; Oh, S.; Wu, H.-G.; Kim, E.; Kim, B.H.; Kim, J.S.; Chang, J.H. Heart substructural dosimetric parameters and risk of cardiac events after definitive chemoradiotherapy for stage III non-small cell lung cancer. Radiother. Oncol. 2020, 152, 126–132. [Google Scholar] [CrossRef]
- Han, C.-B.; Wang, W.-L.; Quint, L.; Xue, J.-X.; Matuszak, M.; Haken, R.T.; Kong, F.-M. (Spring) Pulmonary artery invasion, high-dose radiation, and overall survival in patients with non-small cell lung cancer. Int. J. Radiat. Oncol. 2014, 89, 313–321. [Google Scholar] [CrossRef]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Finn, C.F. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef]
- Nick, T.G.; Campbell, K.M. Logistic regression. Methods Mol. Biol. 2007, 404, 273–301. [Google Scholar]
- Kravchenko, J.; Berry, M.; Arbeev, K.; Lyerly, H.K.; Yashin, A.; Akushevich, I. Cardiovascular comorbidities and survival of lung cancer patients: Medicare data based analysis. Lung Cancer 2015, 88, 85–93. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Oliveira, G.H. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: The unmet need for onco-cardiology. Mayo Clin. Proc. 2016, 91, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; McGale, P.; Taylor, C.W.; Peto, R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005, 6, 557–565. [Google Scholar] [CrossRef]
- Maraldo, M.V.; Brodin, N.P.; Vogelius, I.R.; Aznar, M.C.; Af Rosenschöld, P.M.; Petersen, P.M.; Specht, L. Risk of developing cardio-vascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen-Segarceanu, E.M.; Bos, W.J.W.; Dorresteijn, L.D.; Rensing, B.J.; van der Heyden, J.A.; Vogels, O.J.; Biesma, D.H. Screening Hodgkin lymphoma survivors for radiotherapy induced cardiovascular disease. Cancer Treat. Rev. 2011, 37, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Hotca, A.; Thor, M.; Deasy, J.O.; Rimner, A. Dose to the cardio-pulmonary system and treatment-induced electrocardiogram abnormalities in locally advanced non-small cell lung cancer. Clin. Transl. Radiat. Oncol. 2019, 19, 96–102. [Google Scholar] [CrossRef]
- Tucker, S.L.; Liu, A.; Gomez, D.; Tang, L.L.; Allen, P.; Yang, J.; Liao, Z.; Grosshans, D. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother. Oncol. 2016, 119, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Wolski, M.J.; Bhatnagar, A.; Flickinger, J.C.; Belani, C.P.; Ramalingam, S.; Greenberger, J.S. Multivariate Analysis of Survival, Local Control, and Time to Distant Metastases in Patients with Unresectable Non–Small-Cell Lung Carcinoma Treated with 3-Dimensional Conformal Radiation Therapy with or Without Concurrent Chemotherapy. Clin. Lung Cancer 2005, 7, 100–106. [Google Scholar] [CrossRef]
- Bobbili, P.; Ryan, K.; Duh, M.S.; Dua, A.; Fernandes, A.W.; Pavilack, M.; Gomez, J.E. Treatment patterns and overall survival among patients with unresectable, stage III non-small-cell lung cancer. Futur. Oncol. 2019, 15, 3381–3393. [Google Scholar] [CrossRef] [PubMed]
- Vojtíšek, R. Cardiac toxicity of lung cancer radiotherapy. Rep. Pract. Oncol. Radiother. 2020, 25, 13–19. [Google Scholar] [CrossRef]
- Zhang, T.W.; Snir, J.; Boldt, R.G.; Rodrigues, G.B.; Louie, A.V.; Gaede, S.; McGarry, R.C.; Urbanic, J.J.; Daly, M.E.; Palma, D.A. Is the Importance of Heart Dose Overstated in the Treatment of Non-Small Cell Lung Cancer? A Systematic Review of the Literature. Int. J. Radiat. Oncol. 2019, 104, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Hart, C.N.; Price, G.J.; Faivre-Finn, C.; Aznar, M.C.; Van Herk, M.; Johnson, C.N. Residual setup errors towards the heart after image guidance linked with poorer survival in lung cancer patients: Do we need stricter igrt protocols? Int. J. Radiat. Oncol. 2018, 102, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Taunk, N.K.; Haffty, B.G.; Kostis, J.B.; Egoyal, S. Radiation-Induced Heart Disease: Pathologic Abnormalities and Putative Mechanisms. Front. Oncol. 2015, 5, 39. [Google Scholar] [CrossRef]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-Related Heart Disease: Current Knowledge and Future Prospects. Int. J. Radiat. Oncol. 2010, 76, 656–665. [Google Scholar] [CrossRef]
- Virmani, R.; Farb, A.; Carter, A.J.; Jones, R.M. Pathology of radiation-induced coronary artery disease in human and pig. Cardiovasc. Radiat. Med. 1999, 1, 98–101. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Venkatesulu, B.P.; Mahadevan, L.S.; Krishnan, S. Radiation-Induced Cardiovascular Disease: A Clinical Perspective. Front. Cardiovasc. Med. 2017, 4. [Google Scholar] [CrossRef]
- Leibowitz, A.; Grossman, E.; Berkovitch, A.; Levartovski, M.; Appel, S.; Sharabi, Y.; Gluck, I. The effect of head and neck radiotherapy on blood pressure and orthostatic hypotension in patients with head and neck tumors. Am. J. Hypertens. 2017, 31, 235–239. [Google Scholar] [CrossRef]
| Min | Max | Median | Range | |
|---|---|---|---|---|
| Mean dose to the HB (Gy) | 0.77 (0.80) | 48.48 (47.96) | 17.26 (17.18) | 47.71 (47.17) |
| Maximum dose to the HB (Gy) | 1.79 (1.79) | 81.50 (70.54) | 60.99 (60.99) | 79.71 (68.75) |
| V10 of the HB (%) | 0.00 (0.00) | 100.00 (100.00) | 56.07 (52.61) | 100.00 (100.00) |
| V20 of the HB (%) | 0.00 (0.00) | 100.00 (100.00) | 33.66 (34.33) | 100.00 (100.00) |
| V30 of the HB (%) | 0.00 (0.00) | 95.76 (94.03) | 15.92 (17.87) | 95.76 (94.03) |
| V40 of the HB (%) | 0.00 (0.00) | 76.10 (71.48) | 6.65 (8.02) | 76.10 (71.48) |
| V50 of the HB (%) | 0.00 (0.00) | 49.74 (49.14) | 1.49 (2.06) | 49.74 (49.14) |
| V60 of the HB (%) | 0.00 (0.00) | 24.88 (25.02) | 0.01 (0.02) | 24.88 (25.02) |
| Mean dose to the heart (Gy) | 0.529 | 62.826 | 6.53 | 62.30 |
| Maximum dose to the heart (Gy) | 1.442 | 76.09 | 62.40 | 74.65 |
| V10 of the Heart (%) | 0.00 | 88.16 | 18.93 | 88.16 |
| V20 of the Heart (%) | 0.00 | 58.49 | 8.69 | 58.49 |
| V30 of the Heart (%) | 0.00 | 38.83 | 3.90 | 38.83 |
| V40 of the Heart (%) | 0.00 | 23.91 | 1.91 | 23.91 |
| V50 of the Heart (%) | 0.00 | 10.36 | 0.92 | 10.36 |
| V60 of the Heart (%) | 0.00 | 2.72 | 0.09 | 2.72 |
| Mean dose to the GBV (Gy) | 1.74 | 53.60 | 32.65 | 51.86 |
| Maximum dose to the GBV (Gy) | 12.24 | 84.08 | 63.78 | 71.84 |
| V10 of the GBV (%) | 0.41 | 100.00 | 92.42 | 99.59 |
| V20 of the GBV (%) | 0.00 | 95.40 | 67.35 | 95.40 |
| V30 of the GBV (%) | 0.00 | 87.76 | 47.56 | 87.76 |
| V40 of the GBV (%) | 0.00 | 72.35 | 32.78 | 72.35 |
| V50 of the GBV (%) | 0.00 | 61.41 | 24.20 | 61.41 |
| V60 of the GBV (%) | 0.00 | 50.38 | 12.43 | 50.38 |
| Survived during Follow-Up | Fatal Endpoint | Significance | |
|---|---|---|---|
| Mean dose to the HB (Gy) | 13.32 (5.84) (14.30 (35.60)) | 31.93 (29.31) (31.51 (30.18)) | 0.001 (0.001) |
| Max. dose to the HB (Gy) | 58.95 (79.71) (58.94 (67.62)) | 63.24 (11.34) (63.24 (11.34)) | 0.095 (0.070) |
| V10 of the HB (%) | 43.66 (100.00) (44,12 (100.00)) | 96.26 (63.59) (95.09 (64.72)) | 0.024 (0.021) |
| V20 of the HB (%) | 26.44 (89.55) (26.48 (87.58)) | 71.19 (73.13) (71.46 (76.00)) | 0.003 (0.002) |
| V30 of the HB (%) | 11.15 (71.80) (11.89 (70.06)) | 51.25 (71.16) (48.98 (72.19)) | 0.000 (0.001) |
| V40 of the HB (%) | 3.46 (38.71) (4.14 (36.64)) | 27.92 (66.73) (28.57 (59.90)) | 0.000 (0.000) |
| V50 of the HB (%) | 0.75 (14.18) (0.99 (15.37)) | 13.96 (47.67) (15.14 (46.06)) | 0.001 (0.000) |
| V60 of the HB (%) | 0.00 (5.85) (0.00 (6.34)) | 4.37 (24.88) (5.71 (25.02)) | 0.002 (0.002) |
| Mean dose to the heart (Gy) | 5.52 (62.29) | 8.04 (21.29) | 0.816 |
| Max. dose to the heart (Gy) | 62.28 (74.65) | 62.59 (41.89) | 0.090 |
| V10 of the Heart (%) | 14.50 (81.06) | 27.02 (83.15) | 0.094 |
| V20 of the Heart (%) | 7.36 (55.30) | 11.65 (58.15) | 0.059 |
| V30 of the Heart (%) | 3.17 (33.58) | 5.86 (38.83) | 0.070 |
| V40 of the Heart (%) | 1.49 (15.22) | 4.02 (23.91) | 0.065 |
| V50 of the Heart (%) | 0.72 (6.45) | 2.04 (10.36) | 0.061 |
| V60 of the Heart (%) | 0.05 (2.72) | 0.16 (2.65) | 0.313 |
| Mean dose to the GBV (Gy) | 29.83 (51.86) | 42.09 (19.11) | 0.001 |
| Max. dose to the GBV (Gy) | 63.86 (71.84) | 63.66 (9.17) | 0.913 |
| V10 of the GBV (%) | 83.89 (99.59) | 100.00 (7.88) | 0.001 |
| V20 of the GBV (%) | 62.90 (95.36) | 90.58 (19.79) | 0.000 |
| V30 of the GBV (%) | 41.90 (87.76) | 71.90 (33.82) | 0.001 |
| V40 of the GBV (%) | 28.77 (67.15) | 51.19 (42.71) | 0.001 |
| V50 of the GBV (%) | 21.69 (58.99) | 38.89 (44.55) | 0.002 |
| V60 of the GBV (%) | 12.19 (47.56) | 23.20 (40.85) | 0.04 |
| (A) HB: Nagelkerke R Square = 0.674; Hosmer & Lameshow Test: Chi-Square = 2.563; Sig. = 0.959 GVB: Nagelkerke R Square = 0.638; Hosmer & Lameshow Test: Chi-Square = 1.878; Sig. = 0.985; | ||||
| Coefficient | Standard Error | Sig. | Odds Ratio (95% C.I.) | |
| Age (years): | ||||
| HB | −0.036 | 0.069 | 0.595 | 0.964 (0.843–1.103) |
| GBV | 0.063 | 0.09 | 0.485 | 1.065 (0.893–1.270) |
| Gender: | ||||
| HB | −19,474 | 11,683,949 | 0.999 | 0.000 (0.000) |
| GBV | −23,157 | 9,436,999 | 0.998 | 0.000 (0.000) |
| Chemotherapy: | ||||
| HB | −1.237 | 1.720 | 0.472 | 0.290 (0.010–8.450) |
| GBV | 0.428 | 1.291 | 0.740 | 1.535 (0.122–19.284) |
| Average dose to the HB (Gy) | 0.268 | 0.107 | 0.012 | 1.307 (1.060–1.612) |
| Average dose to the: GBV (Gy) | 0.352 | 0.170 | 0.038 | 1.422 (1.020–1.982) |
| Maximum dose to the HB (Gy) | 0.031 | 0.093 | 0.735 | 1.032 (0.860–1.238) |
| Maximum dose to the GBV (Gy) | −0.024 | 0.194 | 0.901 | 0.976 (0.668–1.428) |
| Average dose to the heart (Gy): | ||||
| HB | −0.124 | 0.118 | 0.293 | 0.884 (0.702–1.113) |
| GBV | 0.007 | 0.040 | 0.866 | 1.007 (0.931–1.089) |
| Maximum dose to the heart (Gy): | ||||
| HB | −0.029 | 0.041 | 0.474 | 0.971 (0.896–1.052) |
| GBV | 0.027 | 0.052 | 0.610 | 1.027 (0.927–1.137) |
| (B) HB: Nagelkerke R Square = 0.546; Hosmer & Lameshow Test: Chi-Square = 6.464; Sig. = 0.595 GVB: Nagelkerke R Square = 0.352; Hosmer & Lameshow Test: Chi-Square = 6.991; Sig. = 0.538 | ||||
| Average dose to the: | ||||
| HB (Gy) | 0.199 | 0.064 | 0.002 | 1.220 (1.076–1.385) |
| GVB (Gy) | 0.147 | 0.056 | 0.008 | 1.158 (1.038–1.292) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steponavičienė, R.; Jonušas, J.; Griškevičius, R.; Venius, J.; Cicėnas, S. A Pilot Study of Safer Radiation Dosage to the Heart and Its Subregions. Medicina 2021, 57, 320. https://doi.org/10.3390/medicina57040320
Steponavičienė R, Jonušas J, Griškevičius R, Venius J, Cicėnas S. A Pilot Study of Safer Radiation Dosage to the Heart and Its Subregions. Medicina. 2021; 57(4):320. https://doi.org/10.3390/medicina57040320
Chicago/Turabian StyleSteponavičienė, Rita, Justinas Jonušas, Romualdas Griškevičius, Jonas Venius, and Saulius Cicėnas. 2021. "A Pilot Study of Safer Radiation Dosage to the Heart and Its Subregions" Medicina 57, no. 4: 320. https://doi.org/10.3390/medicina57040320
APA StyleSteponavičienė, R., Jonušas, J., Griškevičius, R., Venius, J., & Cicėnas, S. (2021). A Pilot Study of Safer Radiation Dosage to the Heart and Its Subregions. Medicina, 57(4), 320. https://doi.org/10.3390/medicina57040320





