SARS-CoV-2 Detection in Fecal Sample from a Patient with Typical Findings of COVID-19 Pneumonia on CT but Negative to Multiple SARS-CoV-2 RT-PCR Tests on Oropharyngeal and Nasopharyngeal Swab Samples
Abstract
1. Introduction
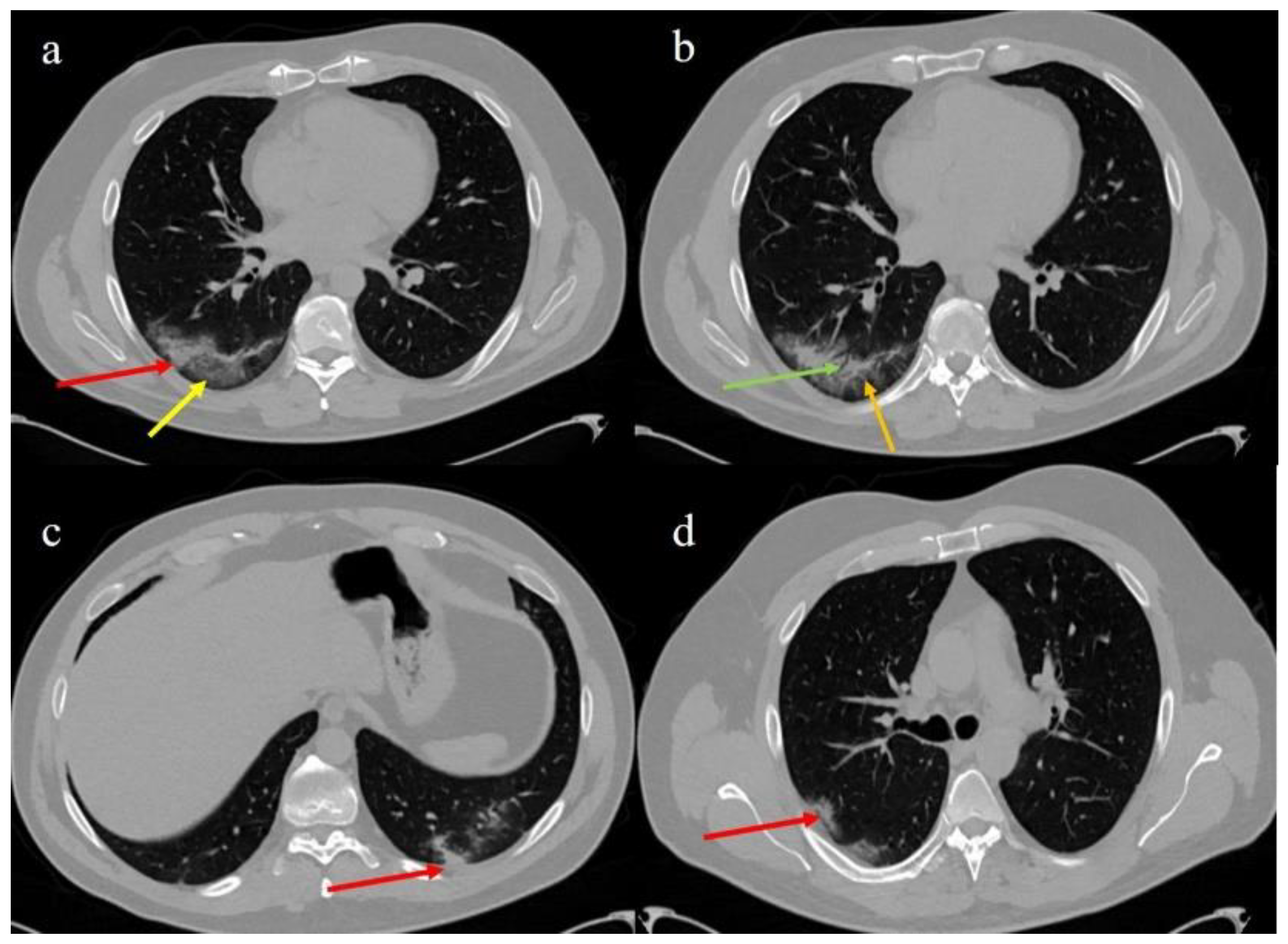
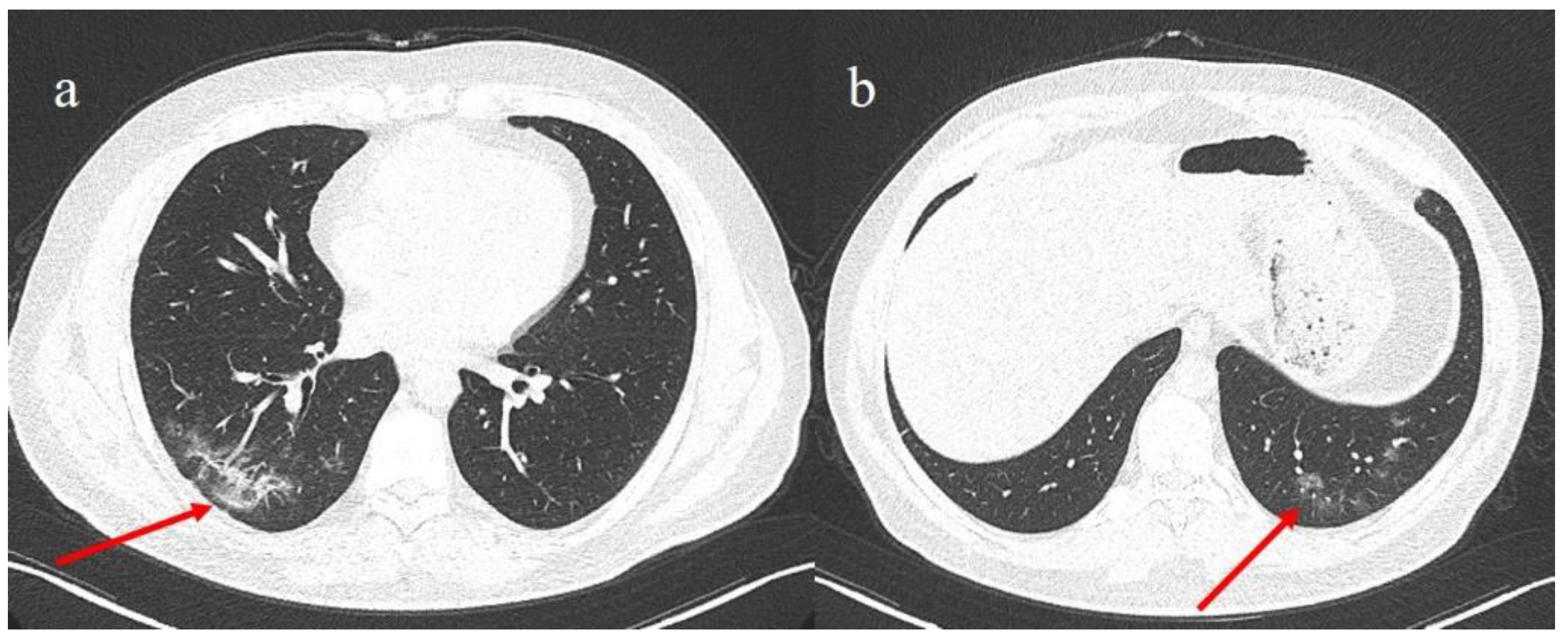
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis. 2020, 94, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Stawicki, S.P.; Jeanmonod, R.; Miller, A.C.; Paladino, L.; Gaieski, D.F.; Yaffee, A.Q.; De Wulf, A.; Grover, J.; Papadimos, T.J.; Yafer, Y.; et al. The 2019–2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: A joint american college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group consensus paper. J. Glob. Infect. Dis. 2020, 12, 47–93. [Google Scholar] [CrossRef]
- Yang, L.; Tu, L. Implications of gastrointestinal manifestations of COVID-19. Lancet Gastroenterol. Hepatol. 2020, 5, 629–630. [Google Scholar] [CrossRef]
- Kopel, J.; Perisetti, A.; Gajendran, M.; Boregowda, U.; Goyal, H. Clinical insights into the gastrointestinal manifestations of COVID-19. Dig. Dis. Sci. 2020, 65, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Petrillo, M.; Brogna, C.; Cristoni, S.; Querci, M.; Piazza, O.; Van den Eede, G. Increase of Sars-Cov2 RNA load in fecal samples prompts for rethinking of Sars-Cov-2 biology and epidemiology. Lancet 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, S.W.; Volpi, A.; Yim, J.J.; Martin, I.B.K.; et al. The role of chest imaging in patient management during the COVID-19 pandemic: A multinational consensus statement from the Fleischner Society. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Brogna, B.; Bignardi, E.; Brogna, C.; Alberigo, M.; Grappone, M.; Musto, L.; Megliola, A.; Salvatore, P.; Fontanella, G. Typical CT findings of COVID-19 pneumonia in patients presenting with repetitive negative RT-PCR. Radiography 2020. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.H.; Li, B.; Zheng, X.S.; Yang, X.L.; Zhou, P.; Ben, H.; Wang, Y.Y.; Xiao, G.F.; Yan, B.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Yang, J.; Liu, B.; Wang, W.; Wei, C. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Kuang, L.; Fang, X.; Mishra, N.; Shan, H. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Kipkorir, V.; Cheruiyot, I.; Ngure, B.; Misiani, M.; Munguti, J. Prolonged SARS-Cov-2 RNA detection in anal/rectal swabs and stool specimens in COVID-19 patients after negative conversion in nasopharyngeal RT-PCR test. J. Med. Virol. 2020, 92, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Long, X.; Ren, C.; He, R.; Yan, X.; Liu, E.; Xu, H.; Li, Q.; Li, W. Follow-up study of long-time positive RT-PCR in stool specimens from asymptomatic children infected with SARS-CoV-2. Pediatr. Infect. Dis. J. 2020, 39, e315–e317. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Xue, Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J. Med. Virol. 2020, 92, 680–682. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, M.; Zou, Z.; Wang, X.; Chen, C.; Qiu, J. Asymptomatic SARS-CoV-2 infected case with viral detection positive in stool but negative in nasopharyngeal samples lasts for 42 days. J. Med. Virol. 2020, 92, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Tong, Z.D.; Wang, H.L.; Dai, Y.X.; Li, K.F.; Liu, J.N.; Wu, W.J.; Yuan, C.; Yu, M.Y.; Li, P.; et al. Detection of novel Coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Infect. Dis. 2020, 26, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Mim, M.A.; Rakhi, N.N.; Saha, O.; Rahaman, M.M. Recommendation of fecal specimen for routine molecular detection of SARS-CoV-2 and for COVID-19 discharge criteria. Pathog. Glob. Health 2020, 114, 168–169. [Google Scholar]
- Szymczak, W.A.; Goldstein, D.Y.; Orner, E.P.; Fecher, R.A.; Yokoda, R.T.; Skalina, K.A.; Fox, A.S.; Gendlina, S. Utility of stool PCR for the diagnosis of COVID-19: Comparison of two commercial platforms. J. Clin. Microbiol. 2020, 58, e01369–e01420. [Google Scholar] [CrossRef]
- Chen, L.D.; Li, H.; Ye, Y.M.; Wu, Z.; Huang, Y.P.; Zhang, W.L.; Li, L. A COVID-19 patient with multiple negative results for PCR assays outside Wuhan, China: A case report. BMC Infect. Dis. 2020, 20, 517. [Google Scholar] [CrossRef]
- Long, C.; Xu, H.; Shen, Q.; Zhang, X.; Fan, B.; Li, H.; Wang, C.; Zeng, B.; Li, Z. Diagnosis of the coronavirus disease (COVID-19): RRT-PCR or CT? Eur. J. Radiol. 2020, 126, 108961. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhong, Z.; Zhao, W.; Zheng, C.; Wang, F.; Liu, J. Chest CT for typical 2019-nCoV pneumonia: Relationship to negative RTPCR testing. Radiology 2020, 296, E41–E45. [Google Scholar] [CrossRef]
- Kim, H.; Hyunsook, H.; Soon, H.Y. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: A meta-analysis. Radiology 2020, 296, E145–E155. [Google Scholar] [CrossRef] [PubMed]
- Ojha, V.; Mani, A.; Pandey, N.N.; Sharma, S.; Kumar, S. CT in coronavirus disease 2019 (COVID-19): A systematic review of chest CT findings in 4410 adult patients. Eur. Radiol. 2020, 30, 6129–6138. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Zheng, C.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT findings in coronavirus disease-19 (COVID-19): Relationship to duration of infection. Radiology 2020, 295, 200463. [Google Scholar] [CrossRef]
- Chung, M.; Bernheim, A.; Mei, X.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Hu, H.; et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-managementpatients.html (accessed on 16 April 2020).
- ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. Available online: https://www.acr.org/Advocacy-and-Economics/ACR-PositionStatements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (accessed on 16 April 2020).
- Long, D.R.; Gombar, S.; Hogan, C.A.; Greninger, A.L.; O’Reilly-Shah, V.; BrysonCahn, C.; Stevens, B.; Rustagi, A.; Jerrome, K.; Kong, K.S.; et al. Occurrence and timing of subsequent SARS-CoV-2 RT-PCR positivity among initially negative patients. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Buitrago-Garcia, D.; Simancas-Racines, D.; Zambrano-Achig, P.; Del Campo, R.; Zamora, J.; Molina, J.A.P.; Low, N.; Bossuyt, P.M. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS ONE 2020, 15, e0242958. [Google Scholar]
- Chen, D.; Xu, W.; Lei, Z.; Huang, Z.; Liu, J.; Gao, Z.; Peng, L. Recurrence of positive SARS-CoV-2 RNA in COVID-19: A case report. Int. J. Infect. Dis. 2020, 93, 297–931. [Google Scholar] [CrossRef]
- Rokkas, T. Gastrointestinal involvement in COVID-19: A systematic review and meta-analysis. Ann. Gaestrenterol. 2020, 33, 355. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.; Chan, P.P.; Lung, K.C.; Tso, E.; Liu, L.W.K.; Ng, Y.Y.; Chu, M.Y.; Chung, T.; Tam, R.; et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: Systematic review and meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Neu, U.; Mainou, B.A. Virus interactions with bacteria: Partners in the infectious dance. PLoS Pathog. 2020, 16, e1008234. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Puthia, M.; Petrlova, J.; Strömdahl, A.C.; Kjellström, S.; Schmidtchen, A. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell. Biol. 2020, 10, mjaa067. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovik, M.; Oliveira, T.; Cipolla, M.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
| Laboratory Parameters | SU | Patient’s Value at Hospital Admission | Normal Value Range |
|---|---|---|---|
| Hemoglobin | mg/dL | 16.0 | 13.0–17.5 |
| Mean cell volume | fL | 89.1 | 80.0–98.0 |
| Platelets count | X1000/μL | 96.0 | 140.00–450.00 |
| White blood count | X1000/μL | 3.00 | 4.00–11.0 |
| Neutrophils | % | 57.6 | 40.0–75.0 |
| Lymphocytes | % | 33.7 | 20.0–50.0 |
| Monocytes | % | 6.1 | 0.0–11.0 |
| Eosinophils | % | 0.4 | 0.0–0.7 |
| Basophiles | 0.6 | 0.0–0.2 | |
| Aspartate transaminases | U/L | 40 | <37 |
| Alanine transaminases | U/L | 44 | <41 |
| Glycemia | mg/dL | 90 | 60–110 |
| Creatinine | mg/dL | 1.01 | 0.7–1.3 |
| Lactate dehydrogenase | U/L | 196 | 135–225 |
| C Reactive Protein | mg/dL | 0.47 | <0.5 |
| D-Dimer | mg/dL | 0.7 | <0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brogna, B.; Brogna, C.; Petrillo, M.; Conte, A.M.; Benincasa, G.; Montano, L.; Piscopo, M. SARS-CoV-2 Detection in Fecal Sample from a Patient with Typical Findings of COVID-19 Pneumonia on CT but Negative to Multiple SARS-CoV-2 RT-PCR Tests on Oropharyngeal and Nasopharyngeal Swab Samples. Medicina 2021, 57, 290. https://doi.org/10.3390/medicina57030290
Brogna B, Brogna C, Petrillo M, Conte AM, Benincasa G, Montano L, Piscopo M. SARS-CoV-2 Detection in Fecal Sample from a Patient with Typical Findings of COVID-19 Pneumonia on CT but Negative to Multiple SARS-CoV-2 RT-PCR Tests on Oropharyngeal and Nasopharyngeal Swab Samples. Medicina. 2021; 57(3):290. https://doi.org/10.3390/medicina57030290
Chicago/Turabian StyleBrogna, Barbara, Carlo Brogna, Mauro Petrillo, Adriana Modestina Conte, Giulio Benincasa, Luigi Montano, and Marina Piscopo. 2021. "SARS-CoV-2 Detection in Fecal Sample from a Patient with Typical Findings of COVID-19 Pneumonia on CT but Negative to Multiple SARS-CoV-2 RT-PCR Tests on Oropharyngeal and Nasopharyngeal Swab Samples" Medicina 57, no. 3: 290. https://doi.org/10.3390/medicina57030290
APA StyleBrogna, B., Brogna, C., Petrillo, M., Conte, A. M., Benincasa, G., Montano, L., & Piscopo, M. (2021). SARS-CoV-2 Detection in Fecal Sample from a Patient with Typical Findings of COVID-19 Pneumonia on CT but Negative to Multiple SARS-CoV-2 RT-PCR Tests on Oropharyngeal and Nasopharyngeal Swab Samples. Medicina, 57(3), 290. https://doi.org/10.3390/medicina57030290







