Natural Plant Extracts and Compounds for Rheumatoid Arthritis Therapy
Abstract
1. Introduction
2. Natural Plant Extract (NPE)
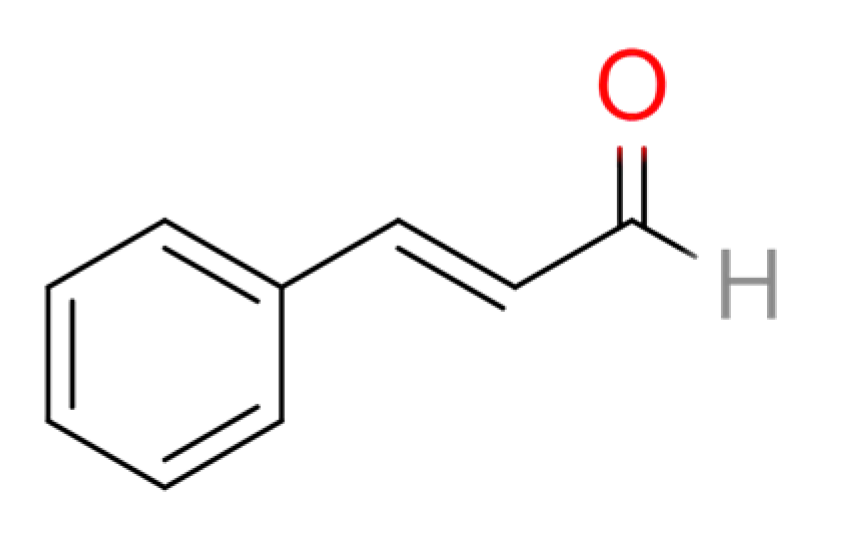
2.1. Cinnamomum cassia Presl
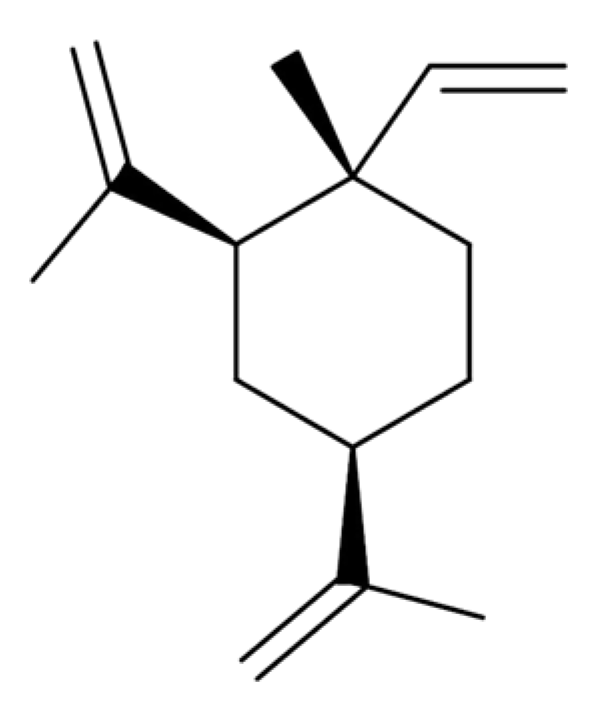
2.2. Ligusticum chuanxiong Hort
2.3. Aconitum kusnezoffii Reichb.
2.4. Tripterygium wilfordii Hook F
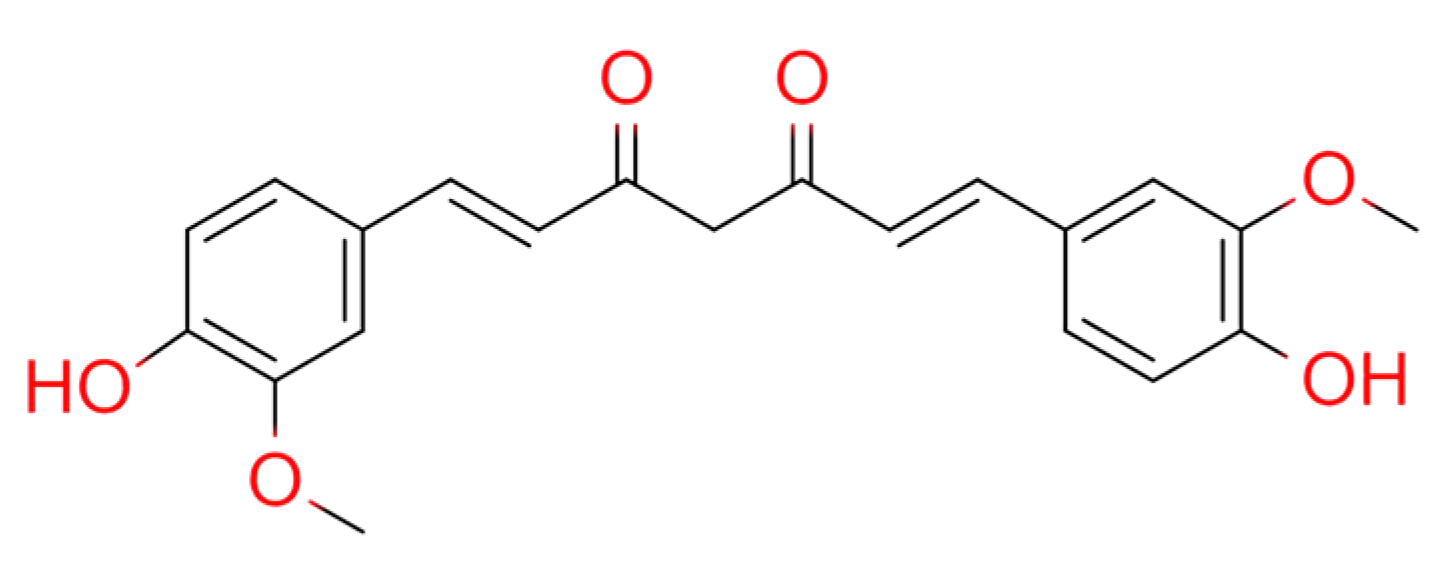
2.5. Curcumae Longae Rhizoma
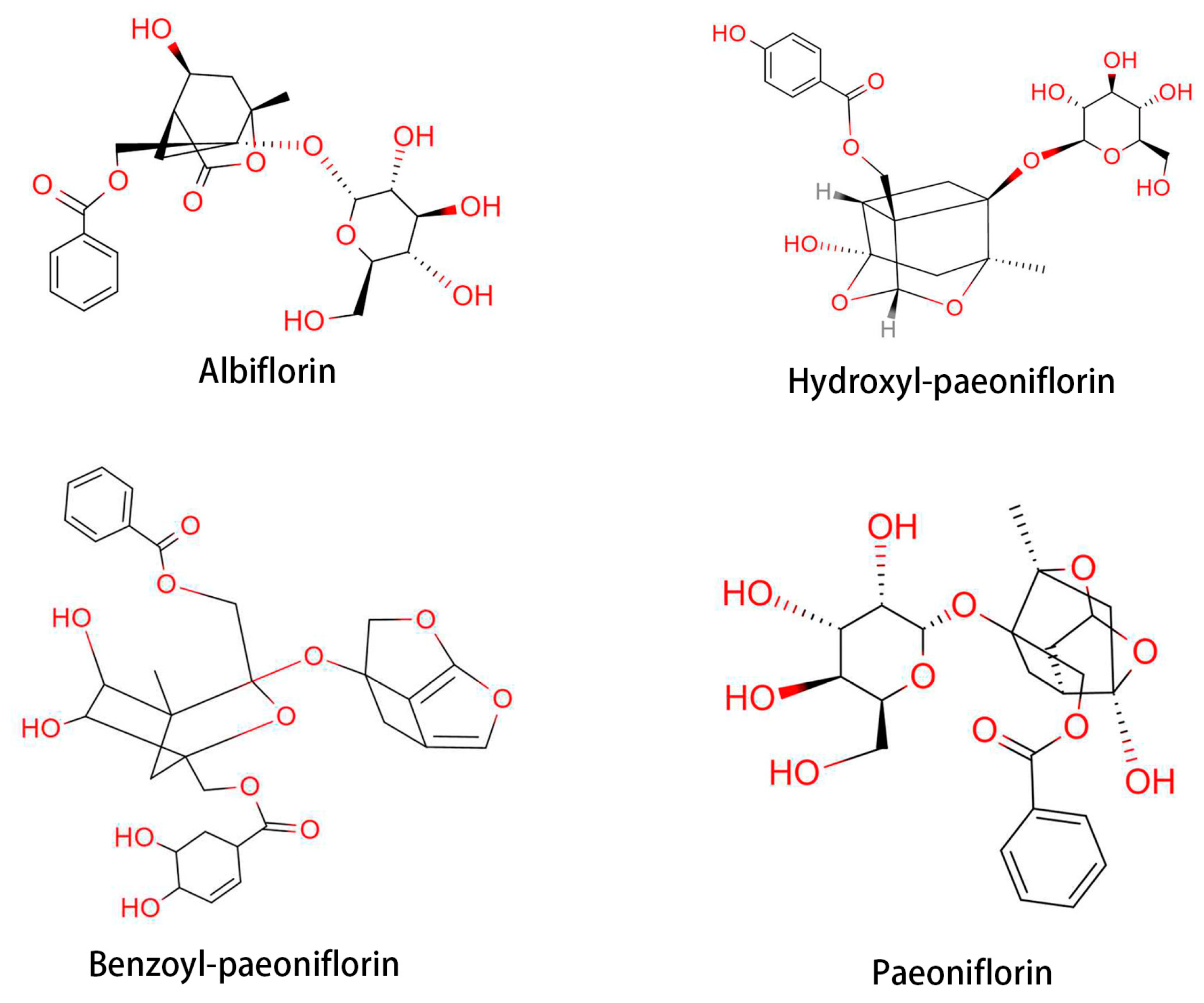
2.6. Paeonia lactiflora Pallas
2.7. Astragalus membranaceus Bunge
2.8. Achyranthes bidentata Blume
3. Treatment of RA with Mixed Herbal Compound
3.1. Wutou Decoction
3.2. GuiZhi ShaoYao ZhiMu Decoction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Petsch, C.; Araujo, E.G.; Englbrecht, M.; Bayat, S.; Cavallaro, A.; Hueber, A.J.; Lell, M.; Schett, G.; Manger, B.; Rech, J. Prevalence of monosodium urate deposits in a population of rheumatoid arthritis patients with hyperuricemia. Semin. Arthritis Rheum. 2016, 45, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Shamriz, O.; Nussinovitch, U.; Rose, N.R. Chapter 1—Pathophysiology of Autoimmunity and Immune-Mediated Mechanisms in Cardiovascular Diseases. In The Heart in Rheumatic, Autoimmune and Inflammatory Diseases; Nussinovitch, U., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 3–23. [Google Scholar] [CrossRef]
- Summers, K.M.; Kockler, D.R. Rituximab treatment of refractory rheumatoid arthritis. Ann. Pharmacother. 2005, 39, 2091–2095. [Google Scholar] [CrossRef]
- Lau, C.S.; Chia, F.; Dans, L.; Harrison, A.; Hsieh, T.Y.; Jain, R.; Jung, S.M.; Kishimoto, M.; Kumar, A.; Leong, K.P.; et al. 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int. J. Rheum. Dis. 2019, 22, 357–375. [Google Scholar] [CrossRef]
- Ferro, F.; Elefante, E.; Luciano, N.; Talarico, R.; Todoerti, M. One year in review 2017: Novelties in the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2017, 35, 721–734. [Google Scholar]
- Xu, T.; Liu, S.; Zhao, J.; Feng, G.; Pi, Z.; Song, F.; Liu, Z. A study on the effective substance of the Wu-tou formula based on the metabonomic method using UPLC-Q-TOF-HDMS. Mol. Biosyst. 2015, 11, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fan, L.; Fan, S.; Wang, J.; Luo, T.; Tang, Y.; Chen, Z.; Yu, L. Cinnamomum cassia Presl: A Review of Its Traditional Uses, Phytochemistry, Pharmacology and Toxicology. Molecules 2019, 24, 3473. [Google Scholar] [CrossRef]
- Yang, Y.L.; Luo, B.; Zhang, H.; Zheng, W.J.; Wu, M.L.; Li, S.Y.; Gao, H.Y.; Li, Q.; Ge, Y.W.; Yang, Q. Advances in quality research of Cinnamomum cassia. Zhongguo Zhong Yao Za Zhi 2020, 45, 2792–2799. [Google Scholar] [PubMed]
- Sun, L.; Zong, S.B.; Li, J.C.; Lv, Y.Z.; Liu, L.N.; Wang, Z.Z.; Zhou, J.; Cao, L.; Kou, J.P.; Xiao, W. The essential oil from the twigs of Cinnamomum cassia Presl alleviates pain and inflammation in mice. J. Ethnopharmacol. 2016, 194, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, J.; Wen, W.; Pan, T.; Chen, H.; Fu, Y.; Wang, F.; Huang, J.H.; Xu, S. Cinnamaldehyde suppresses NLRP3 derived IL-1β via activating succinate/HIF-1 in rheumatoid arthritis rats. Int. Immunopharmacol. 2020, 84, 106570. [Google Scholar] [CrossRef] [PubMed]
- Braddock, M.; Quinn, A.; Canvin, J. Therapeutic potential of targeting IL-1 and IL-18 in inflammation. Expert Opin. Biol. Ther. 2004, 4, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Rehman, M.T.; Shahzad, S.; Naeem, S.S.; Faizy, A.F.; Khan, A.Q.; Khan, M.S.; Husain, F.M.; Moin, S. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur. J. Pharmacol. 2019, 852, 14–24. [Google Scholar] [CrossRef]
- Li, W.; Tang, Y.; Chen, Y.; Duan, J.A. Advances in the chemical analysis and biological activities of chuanxiong. Molecules 2012, 17, 10614–10651. [Google Scholar] [CrossRef]
- Yuan, X.; Han, B.; Feng, Z.M.; Jiang, J.S.; Yang, Y.N.; Zhang, P.C. Chemical constituents of Ligusticum chuanxiong and their anti-inflammation and hepatoprotective activities. Bioorg. Chem. 2020, 101, 104016. [Google Scholar] [CrossRef]
- Du, J.C.; Xie, X.F.; Xiong, L.; Sun, C.; Peng, C. Research progress of chemical constituents and pharmacological activities of essential oil of Ligusticum chuanxiong. Zhongguo Zhong Yao Za Zhi 2016, 41, 4328–4333. [Google Scholar]
- Huang, C.; Cao, X.; Chen, X.; Fu, Y.; Zhu, Y.; Chen, Z.; Luo, Q.; Li, L.; Song, X.; Jia, R.; et al. A pectic polysaccharide from Ligusticum chuanxiong promotes intestine antioxidant defense in aged mice. Carbohydr. Polym. 2017, 174, 915–922. [Google Scholar] [CrossRef]
- Mu, C.X.; Liu, G.L.; Tian, H.; Li, Y.C.; Huang, Y.L. Effect of Tetramethyl pyrazine on serum levels of IL-1beta, IL-6, and IL-2, and NO and PGE2 in the synovial fluid of CIA rats: An experimental research. Zhongguo Zhong Xi Yi Jie He Za Zhi 2014, 34, 214–217. [Google Scholar]
- Zhang, C.; Guan, D.; Jiang, M.; Liang, C.; Li, L.; Zhao, N.; Zha, Q.; Zhang, W.; Lu, C.; Zhang, G.; et al. Efficacy of leflunomide combined with ligustrazine in the treatment of rheumatoid arthritis: Prediction with network pharmacology and validation in a clinical trial. Chin. Med. 2019, 14, 26. [Google Scholar] [CrossRef]
- Sun, H.; Wang, M.; Zhang, A.; Ni, B.; Dong, H.; Wang, X. UPLC-Q-TOF-HDMS analysis of constituents in the root of two kinds of Aconitum using a metabolomics approach. Phytochem. Anal. 2013, 24, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Jin, B.; Li, Y.; Wang, F.; Yang, Y.; Cui, Y.; Song, X.; Yue, Z.; Liu, J. C19-Norditerpenoid Alkaloids from Aconitum szechenyianum. Molecules 2018, 23, 1108. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Li, M.; Li, Y.B.; Lei, B.B.; Yuan, X.; Xing, X.K.; Xie, Y.F.; Wang, M.; Wang, L.; Yang, H.J.; et al. Benzoylaconitine Inhibits Production of IL-6 and IL-8 via MAPK, Akt, NF-κB Signaling in IL-1β-Induced Human Synovial Cells. Biol. Pharm. Bull. 2020, 43, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.; Hao, X.; Zhao, J.; Wang, L.; Liu, J.; Jiang, H.; Jin, H.; Liu, G.; Feng, Y. Delivery of benzoylaconitine using biodegradable nanoparticles to suppress inflammation via regulating NF-κB signaling. Colloids Surf. B Biointerfaces 2020, 191, 110980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Lin, Z.J.; Zhang, B.; Li, A. Traditional prescription rules of Aconitum herbs in treatment of Bi syndrome. Zhongguo Zhong Yao Za Zhi 2018, 43, 211–215. [Google Scholar] [PubMed]
- Bao, J.; Dai, S.M. A Chinese herb Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis: Mechanism, efficacy, and safety. Rheumatol. Int. 2011, 31, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Xie, F.G.; Yang, J.Z.; Luo, Y.M.; Chen, X.G.; Zhang, D.M. Two sesquiterpene pyridine alkaloids and a triterpenoid saponin from the root barks of Tripterygium hypoglaucum. J. Asian Nat. Prod. Res. 2012, 14, 973–980. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.J.; Yang, J.Z.; Ma, J.; Chen, X.G.; Hou, Q.; Zhang, D.M. Anti-inflammatory sesquiterpene derivatives from the leaves of Tripterygium wilfordii. J. Nat. Prod. 2013, 76, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Zhao, L.D.; Chen, H.; Zhang, Y.; Wang, D.F.; Huang, L.F.; Lv, Q.W.; Liu, B.; Li, Z.; Wei, W.; et al. Comparison of the impact of Tripterygium wilfordii Hook F and Methotrexate treatment on radiological progression in active rheumatoid arthritis: 2-year follow up of a randomized, non-blinded, controlled study. Arthritis Res. Ther. 2018, 20, 70. [Google Scholar] [CrossRef]
- Fan, D.; Guo, Q.; Shen, J.; Zheng, K.; Lu, C.; Zhang, G.; Lu, A.; He, X. The Effect of Triptolide in Rheumatoid Arthritis: From Basic Research towards Clinical Translation. Int. J. Mol. Sci. 2018, 19, 376. [Google Scholar] [CrossRef]
- Tong, L.; Nanjundaiah, S.M.; Venkatesha, S.H.; Astry, B.; Yu, H.; Moudgil, K.D. Pristimerin, a naturally occurring triterpenoid, protects against autoimmune arthritis by modulating the cellular and soluble immune mediators of inflammation and tissue damage. Clin. Immunol. 2014, 155, 220–230. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, X.; Wang, D.; Li, M.; Liu, Z. Triptolide protects bone against destruction by targeting RANKL-mediated ERK/AKT signalling pathway in the collagen-induced rheumatoid arthritis. Biomed. Res. 2017, 28, 4111–4116. [Google Scholar]
- Liu, C.-f.; Lin, N.; Jia, H.-w.; Xiao, C.; Zhang, L. Effect of Triptolide on Immunological Function of Mice with Collagen II-induced Arthritis. Chin. J. Inf. TCM 2004, 7, 602–604. [Google Scholar]
- Wang, J.; Wang, A.; Zeng, H.; Liu, L.; Jiang, W.; Zhu, Y.; Xu, Y. Effect of triptolide on T-cell receptor beta variable gene mRNA expression in rats with collagen-induced arthritis. Anat. Rec. 2012, 295, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Astry, B.; Venkatesha, S.H.; Laurence, A.; Christensen-Quick, A.; Garzino-Demo, A.; Frieman, M.B.; O’Shea, J.J.; Moudgil, K.D. Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin. Immunol. 2015, 157, 228–238. [Google Scholar] [CrossRef]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef]
- Turmeric. In Drugs and Lactation Database (LactMed); National Library of Medicine (US): Bethesda, MD, USA, 2006.
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Mau, J.-L.; Lai, E.Y.C.; Wang, N.-P.; Chen, C.-C.; Chang, C.-H.; Chyau, C.-C. Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem. 2003, 82, 583–591. [Google Scholar] [CrossRef]
- Wilson, B.; Abraham, G.; Manju, V.S.; Mathew, M.; Vimala, B.; Sundaresan, S.; Nambisan, B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J. Ethnopharmacol. 2005, 99, 147–151. [Google Scholar] [CrossRef]
- Angel, G.R.; Menon, N.; Vimala, B.; Nambisan, B. Essential oil composition of eight starchy Curcuma species. Ind. Crops Prod. 2014, 60, 233–238. [Google Scholar] [CrossRef]
- Sundar Dhilip Kumar, S.; Houreld, N.N.; Abrahamse, H. Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules 2018, 23, 835. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Jang, E.; Lee, J.H. Preclinical Evidence of Curcuma longa and Its Noncurcuminoid Constituents against Hepatobiliary Diseases: A Review. Evid. Based Complement. Altern. Med. 2020, 2020, 8761435. [Google Scholar] [CrossRef]
- Zou, S.; Wang, C.; Cui, Z.; Guo, P.; Meng, Q.; Shi, X.; Gao, Y.; Yang, G.; Han, Z. β-Elemene induces apoptosis of human rheumatoid arthritis fibroblast-like synoviocytes via reactive oxygen species-dependent activation of p38 mitogen-activated protein kinase. Pharmacol. Rep. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Chen, J.J.; Dai, L.; Zhao, L.X.; Zhu, X.; Cao, S.; Gao, Y.J. Intrathecal curcumin attenuates pain hypersensitivity and decreases spinal neuroinflammation in rat model of monoarthritis. Sci. Rep. 2015, 5, 10278. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Ru-bing, Y. The Effect of Curcumin on the Immune Organ’s Weight and Expression of TNF-± IL-1² in Blood Serum of Adjuvant Arthritis Rats. Chin. Arch. Tradit. Chin. Med. 2009, 27, 1803–1805. [Google Scholar]
- Mohammadian Haftcheshmeh, S.; Khosrojerdi, A.; Aliabadi, A.; Lotfi, S.; Mohammadi, A.; Momtazi-Borojeni, A.A. Immunomodulatory Effects of Curcumin in Rheumatoid Arthritis: Evidence from Molecular Mechanisms to Clinical Outcomes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–29. [Google Scholar] [CrossRef]
- Zhao, Q.L.; Bian, X.K.; Qian, D.W.; Zhang, T.; Zhu, Z.H.; Guo, S.; Yan, H.; Wang, T.J.; Chen, Z.P.; Duan, J.A. Comparative study on differences of Paeonia lactiflora from different habitats based on fingerprint and chemometrics. Zhongguo Zhong Yao Za Zhi 2019, 44, 3316–3322. [Google Scholar] [PubMed]
- Ma, X.; Chi, Y.H.; Niu, M.; Zhu, Y.; Zhao, Y.L.; Chen, Z.; Wang, J.B.; Zhang, C.E.; Li, J.Y.; Wang, L.F.; et al. Metabolomics Coupled with Multivariate Data and Pathway Analysis on Potential Biomarkers in Cholestasis and Intervention Effect of Paeonia lactiflora Pall. Front. Pharmacol. 2016, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, S.M. Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int. Immunopharmacol. 2012, 14, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, J.; Wang, L.; Wang, S.; Nie, X.; Chen, Y.; Fu, Q.; Jiang, M.; Fu, C.; He, Y. Total glucosides of paeony: A review of its phytochemistry, role in autoimmune diseases, and mechanisms of action. J. Ethnopharmacol. 2020, 258, 112913. [Google Scholar] [CrossRef]
- Du, J.H.; Dong, B.D. Comparative study on clinical efficacy of using methotrexate singly or combined with total glucosides of Paeony in treating rheumatoid arthritis. Zhongguo Zhong xi yi jie he za zhi = Chin. J. Integr. Tradit. Western Med. 2005, 25, 540–542. [Google Scholar]
- Lin, J.; Xiao, L.; Ouyang, G.; Shen, Y.; Huo, R.; Zhou, Z.; Sun, Y.; Zhu, X.; Zhang, J.; Shen, B.; et al. Total glucosides of paeony inhibits Th1/Th17 cells via decreasing dendritic cells activation in rheumatoid arthritis. Cell. Immunol. 2012, 280, 156–163. [Google Scholar] [CrossRef]
- Xu, X.-x.; Qi, X.-M.; Zhang, W.; Zhang, C.-Q.; Wu, X.-X.; Wu, Y.-G.; Wang, K.; Shen, J.-J. Effects of total glucosides of paeony on immune regulatory toll-like receptors TLR2 and 4 in the kidney from diabetic rats. Phytomedicine 2014, 21, 815–823. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wei, W.; Wang, N.P.; Wang, Q.T.; Chen, J.Y.; Chen, Y.; Wu, H.; Hu, X.Y. Paeoniflorin suppresses inflammatory mediator production and regulates G protein-coupled signaling in fibroblast-like synoviocytes of collagen induced arthritic rats. Inflamm. Res. 2008, 57, 388–395. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wei, W.; Wang, Q.T.; Chen, J.Y.; Chen, Y. Cross-talk between MEK1/2-ERK1/2 signaling and G protein-couple signaling in synoviocytes of collagen-induced arthritis rats. Chin. Med. J. 2008, 121, 2278–2283. [Google Scholar] [CrossRef]
- Jia, X.Y.; Chang, Y.; Sun, X.J.; Wei, F.; Wu, Y.J.; Dai, X.; Xu, S.; Wu, H.X.; Wang, C.; Yang, X.Z.; et al. Regulatory effects of paeoniflorin-6′-O-benzene sulfonate (CP-25) on dendritic cells maturation and activation via PGE2-EP4 signaling in adjuvant-induced arthritic rats. Inflammopharmacology 2019, 27, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, C.C.; Cui, D.; Luo, T.T.; Li, Y.; Zhang, Y.; Ma, Y.; Wei, W. Immunomodulatory Effects of CP-25 on Splenic T Cells of Rats with Adjuvant Arthritis. Inflammation 2018, 41, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qi, H.; Jiang, D.; Wang, R.; Chen, A.; Yan, Z.; Xiao, J. The new use of an ancient remedy: A double-blinded randomized study on the treatment of rheumatoid arthritis. Am. J. Chin. Med. 2013, 41, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Y.; Chang, Y.; Sun, X.J.; Wu, H.X.; Wang, C.; Xu, H.M.; Zhang, L.; Zhang, L.L.; Zheng, Y.Q.; Song, L.H.; et al. Total glucosides of paeony inhibit the proliferation of fibroblast-like synoviocytes through the regulation of G proteins in rats with collagen-induced arthritis. Int. Immunopharmacol. 2014, 18, 1–6. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, W.; Zheng, Y.Q.; Jia, X.Y. Effects and mechanisms of total glucosides of paeony on joint damage in rat collagen-induced arthritis. Inflamm. Res. 2005, 54, 211–220. [Google Scholar] [CrossRef]
- Liu, P.W.L.; Tian, M.; Zhang, S.-F.; Cao, G.-H.; Jin, Y.-F.; Liu, J.-F.; Liu, C.-H. Effects of TGP on the Expressions of IL-6,IL-1 and TNF-α in Children with Juvenile Idiopathic Arthritis. Rheum. Arthritis 2020, 009, 10–13. [Google Scholar]
- Chen, Z.; Li, X.P.; Li, Z.J.; Xu, L.; Li, X.M. Reduced hepatotoxicity by total glucosides of paeony in combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis. Int. Immunopharmacol. 2013, 15, 474–477. [Google Scholar] [CrossRef]
- Ding, Z.X.; Yang, S.F.; Wu, Q.F.; Lu, Y.; Chen, Y.Y.; Nie, X.L.; Jie, H.Y.; Qi, J.M.; Wang, F.S. [Therapeutic effect of total glucosides of paeony on lupus nephritis in MRL/lpr mice]. Nan Fang Yi Ke Da Xue Xue Bao 2011, 31, 656–660. [Google Scholar]
- Yu, C.; Fan, X.; Li, Z.; Liu, X.; Wang, G. Efficacy and safety of total glucosides of paeony combined with acitretin in the treatment of moderate-to-severe plaque psoriasis: A double-blind, randomised, placebo-controlled trial. Eur. J. Dermatol. 2017, 27, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Hou, P.; Xiao, W.G. Clinical observation on effect of total glucosides of paeony in treating patients with non-systemic involved Sjögren syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007, 27, 596–598. [Google Scholar] [PubMed]
- Zhang, L.J.; Liu, H.K.; Hsiao, P.C.; Kuo, L.M.; Lee, I.J.; Wu, T.S.; Chiou, W.F.; Kuo, Y.H. New isoflavonoid glycosides and related constituents from astragali radix (Astragalus membranaceus ) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef]
- Song, J.Z.; Yiu, H.H.; Qiao, C.F.; Han, Q.B.; Xu, H.X. Chemical comparison and classification of Radix Astragali by determination of isoflavonoids and astragalosides. J. Pharm. Biomed. Anal. 2008, 47, 399–406. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yang, X.; Wei, J.R.; Chen, N.M.; Xu, J.P.; Bi, Y.Q.; Yang, M.; Gong, X.; Li, Z.Y.; Ren, K.; et al. Ethnopharmacology, Phytochemistry, Pharmacology, Toxicology and Clinical Applications of Radix Astragali. Chin. J. Integr. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Shabbir, A.; Wojcikowski, K.; Wohlmuth, H.; Gobe, G.C. The Antioxidant Effects of Radix Astragali (Astragalus membranaceus and Related Species) in Protecting Tissues from Injury and Disease. Curr. Drug Targets 2016, 17, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Yu, Q.T.; Li, P.; Li, S.L.; Wang, Y.X.; Sheng, L.H.; Yi, L. Quality evaluation of Radix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. J. Chromatogr. A 2006, 1134, 162–169. [Google Scholar] [CrossRef]
- Xiao, H.B.; Krucker, M.; Albert, K.; Liang, X.M. Determination and identification of isoflavonoids in Radix astragali by matrix solid-phase dispersion extraction and high-performance liquid chromatography with photodiode array and mass spectrometric detection. J. Chromatogr. A 2004, 1032, 117–124. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Sang, R.; Yu, Y.; Ge, B.; Zhang, X. Immunomodulatory and anti-inflammatory effects of total flavonoids of Astragalus by regulating NF-ΚB and MAPK signalling pathways in RAW 264.7 macrophages. Pharmazie 2018, 73, 589–593. [Google Scholar] [PubMed]
- Liu, X.Y.; Xu, L.; Wang, Y.; Li, J.X.; Zhang, Y.; Zhang, C.; Wang, S.S.; Zhang, X.M. Protective effects of total flavonoids of Astragalus against adjuvant-induced arthritis in rats by regulating OPG/RANKL/NF-κB pathway. Int. Immunopharmacol. 2017, 44, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Qiong, W.; You-bin, L. Studies on chemical constituents and pharmaceutics activity of Achyranthes bidentata BI. Strait Pharm. J. 2011, 23, 1–6. [Google Scholar]
- Xuelian, S.; Yuan, L.; Honghai, Z. Effect of Total Saponins of Achyranthes on Knee Osteoarthritis Rabbit Cartilage Histomorphology and Contents of IL-1β and TGF-β1 in Synovial Fluid. Tradit. Chin. Drug Res. Clin. Pharmacol. 2016, 27, 14–19. [Google Scholar]
- Zheng, W.; Lu, X.; Fu, Z.; Zhang, L.; Li, X.; Xu, X.; Ren, Y.; Lu, Y.; Fu, H.; Tian, J. Identification of candidate synovial membrane biomarkers after Achyranthes aspera treatment for rheumatoid arthritis. Biochim. Biophys. Acta 2016, 1864, 308–316. [Google Scholar] [CrossRef]
- Hilvo, M.; Baranauskiene, L.; Salzano, A.M.; Scaloni, A.; Matulis, D.; Innocenti, A.; Scozzafava, A.; Monti, S.M.; Di Fiore, A.; De Simone, G.; et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J. Biol. Chem. 2008, 283, 27799–27809. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Maosheng, Q.; Wei, X. Experimental Study of Guizhi Shaoyao Zhimu Decoction on Gene Regulation of Synovial Cell Apoptosis in Rheumatoid Arthritis. Contemp. Med. 2010, 16, 18–20. [Google Scholar]
- Guo, Q.; Mao, X.; Zhang, Y.; Meng, S.; Xi, Y.; Ding, Y.; Zhang, X.; Dai, Y.; Liu, X.; Wang, C.; et al. Guizhi-Shaoyao-Zhimu decoction attenuates rheumatoid arthritis partially by reversing inflammation-immune system imbalance. J. Transl. Med. 2016, 14, 165. [Google Scholar] [CrossRef]
- Feng, C.; Chen, R.; Wang, K.; Wen, C.; Xu, Z. Chinese traditional medicine (GuiZhi-ShaoYao-ZhiMu decoction) as an add-on medication to methotrexate for rheumatoid arthritis: A meta-analysis of randomized clinical trials. Ther. Adv. Chronic Dis. 2021, 12, 2040622321993438. [Google Scholar] [CrossRef]
- Daily, J.W.; Zhang, T.; Cao, S.; Park, S. Efficacy and Safety of GuiZhi-ShaoYao-ZhiMu Decoction for Treating Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Altern. Complement. Med. 2017, 23, 756–770. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Kim, Y.-R.; Min, Y.; Zhao, Y.; Do, K.; Son, Y.-O. Natural Plant Extracts and Compounds for Rheumatoid Arthritis Therapy. Medicina 2021, 57, 266. https://doi.org/10.3390/medicina57030266
Zhao X, Kim Y-R, Min Y, Zhao Y, Do K, Son Y-O. Natural Plant Extracts and Compounds for Rheumatoid Arthritis Therapy. Medicina. 2021; 57(3):266. https://doi.org/10.3390/medicina57030266
Chicago/Turabian StyleZhao, Xiangyu, Young-Rok Kim, Yunhui Min, Yaping Zhao, Kyoungtag Do, and Young-Ok Son. 2021. "Natural Plant Extracts and Compounds for Rheumatoid Arthritis Therapy" Medicina 57, no. 3: 266. https://doi.org/10.3390/medicina57030266
APA StyleZhao, X., Kim, Y.-R., Min, Y., Zhao, Y., Do, K., & Son, Y.-O. (2021). Natural Plant Extracts and Compounds for Rheumatoid Arthritis Therapy. Medicina, 57(3), 266. https://doi.org/10.3390/medicina57030266






