Botulinum Toxin A Injection for the Treatment of Intractable Dry Eye Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N.; et al. TFOS DEWS II Introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A. Dry eye disease: Impact on quality of life and vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef]
- Nigam, P.K.; Nigam, A. Botulinum toxin. Indian J. Dermatol. 2010, 55, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, F.; Pignataro, D.; Lavano, M.A.; Santella, B.; Folliero, V.; Zannella, C.; Astarita, C.; Gagliano, C.; Franci, G.; Avitabile, T.; et al. Current Evidence on the Ocular Surface Microbiota and Related Diseases. Microorganisms 2020, 8, 1033. [Google Scholar] [CrossRef] [PubMed]
- Spiera, H.; Asbell, P.A.; Simpson, D.M. Botulinum toxin increases tearing in patients with Sjögren’s syndrome: A preliminary report. J. Rheumatol. 1997, 24, 1842–1843. [Google Scholar] [PubMed]
- Sahlin, S.; Chen, E.; Kaugesaar, T.; Almqvist, H.; Kjellberg, K.; Lennerstrand, G. Effect of eyelid botulinum toxin injection on lacrimal drainage. Am. J. Ophthalmol. 2000, 129, 481–486. [Google Scholar] [CrossRef]
- Chun, Y.S.; Kim, J.C. Treatment of superior limbic keratoconjunctivitis with a large-diameter contact lens and Botulium Toxin, A. Cornea 2009, 28, 752–758. [Google Scholar]
- Choi, M.G.; Yeo, J.H.; Kang, J.W.; Chun, Y.S.; Lee, J.K.; Kim, J.C. Effects of botulinum toxin type A on the treatment of dry eye disease and tear cytokines. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Serna-Ojeda, J.C.; Nava-Castaneda, A. Paralysis of the orbicularis muscle of the eye using botulinum toxin type A in the treatment for dry eye. Acta Ophthalmol. 2017, 95, e132–e137. [Google Scholar] [CrossRef]
- Horwath-Winter, J.; Bergloeff, J.; Floegel, I.; Haller-Schober, E.M.; Schmut, O. Botulinum toxin A treatment in patients suffering from blepharospasm and dry eye. Br. J. Ophthalmol. 2003, 87, 54–56. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Lee, S.Y.; Yoon, J.S. Meibomian gland dysfunction in longstanding prosthetic eye wearers. Br. J. Ophthalmol. 2013, 97, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, A.A. Botulinum neurotoxin type A versus punctal plug insertion in the management of dry eye disease. Oman J. Ophthalmol. 2014, 7, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Park, D.I.; Shin, H.M.; Lee, S.Y.; Lew, H. Tear production and drainage after botulinum toxin A injection in patients with essential blepharospasm. Acta Ophthalmol. 2013, 91, e108–e112. [Google Scholar] [CrossRef]
- Fouda, S.M.; Mattout, H.K. Comparison between botulinum toxin A injection and lacrimal punctal plugs for the control of post-LASIK dry eye manifestations: A prospective study. Ophthalmol. Ther. 2017, 6, 167–174. [Google Scholar] [CrossRef]
- Isshiki, Y.; Ishikawa, H.; Mimura, O. Changes in ocular higher-order aberrations following botulinum toxin treatment in patients with blepharospasm: BTX improves dry eye in patients with BEB. Jpn. J. Ophthalmol. 2016, 60, 486–491. [Google Scholar] [CrossRef]
- Ho, M.C.; Hsu, W.C.; Hsieh, Y.T. Botulinum toxin type A injection for lateral canthal rhytids: Effect on tear film stability and tear production. JAMA Ophthalmol. 2014, 132, 332–337. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Nichols, K.K.; Mitchell, G.L.; Zadnik, K. The repeatability of clinical measurements of dry eye. Cornea 2004, 23, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Gumus, K.; Lee, S.; Yen, M.T.; Pflugfelder, S.C. Botulinum toxin injection for the management of refractory filamentary keratitis. Arch. Ophthalmol. 2012, 130, 446–450. [Google Scholar] [PubMed]
- Bourkiza, R.; Lee, V. A review of the complications of lacrimal occlusion with punctal and canalicular plugs. Orbit 2012, 31, 86–93. [Google Scholar] [CrossRef] [PubMed]
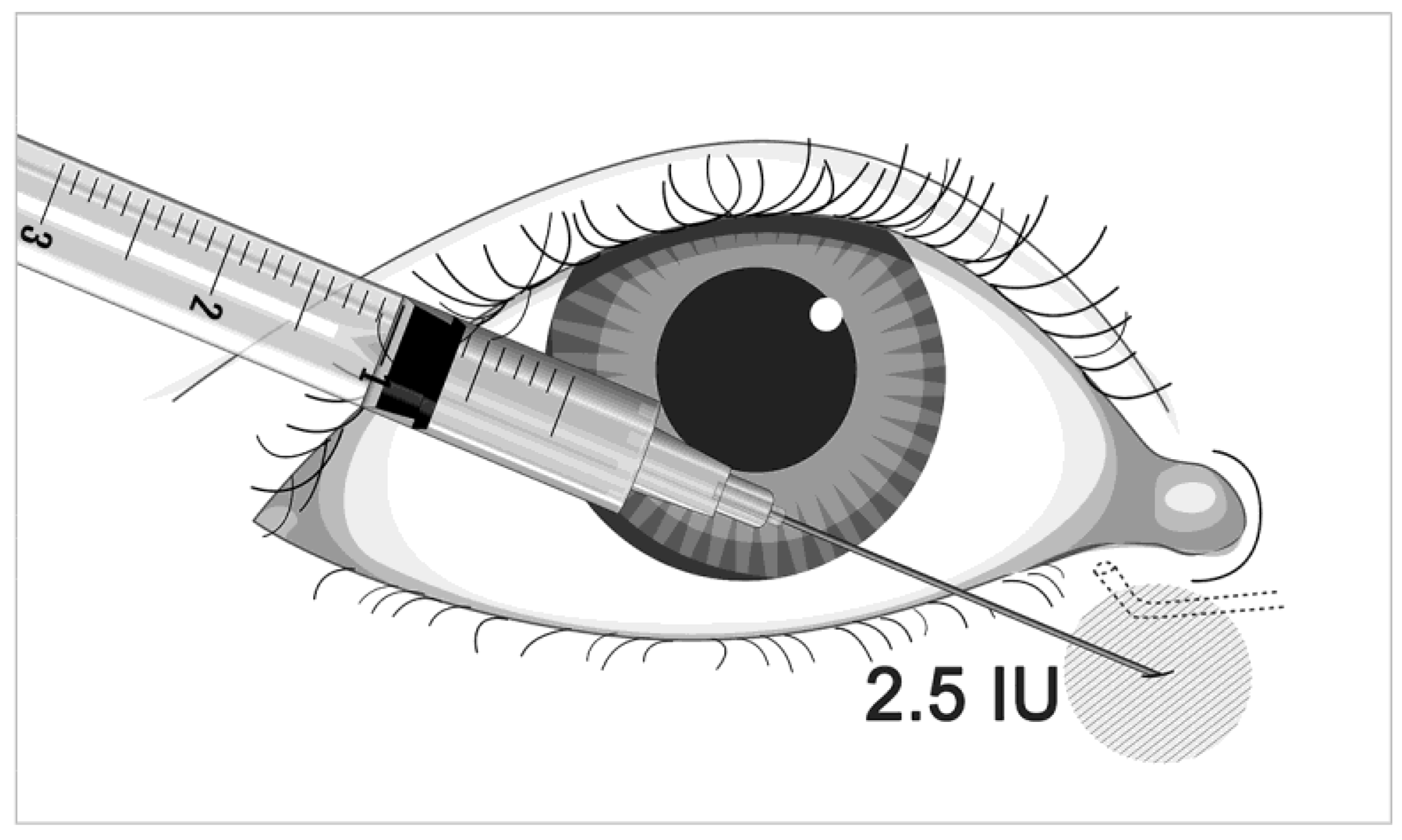
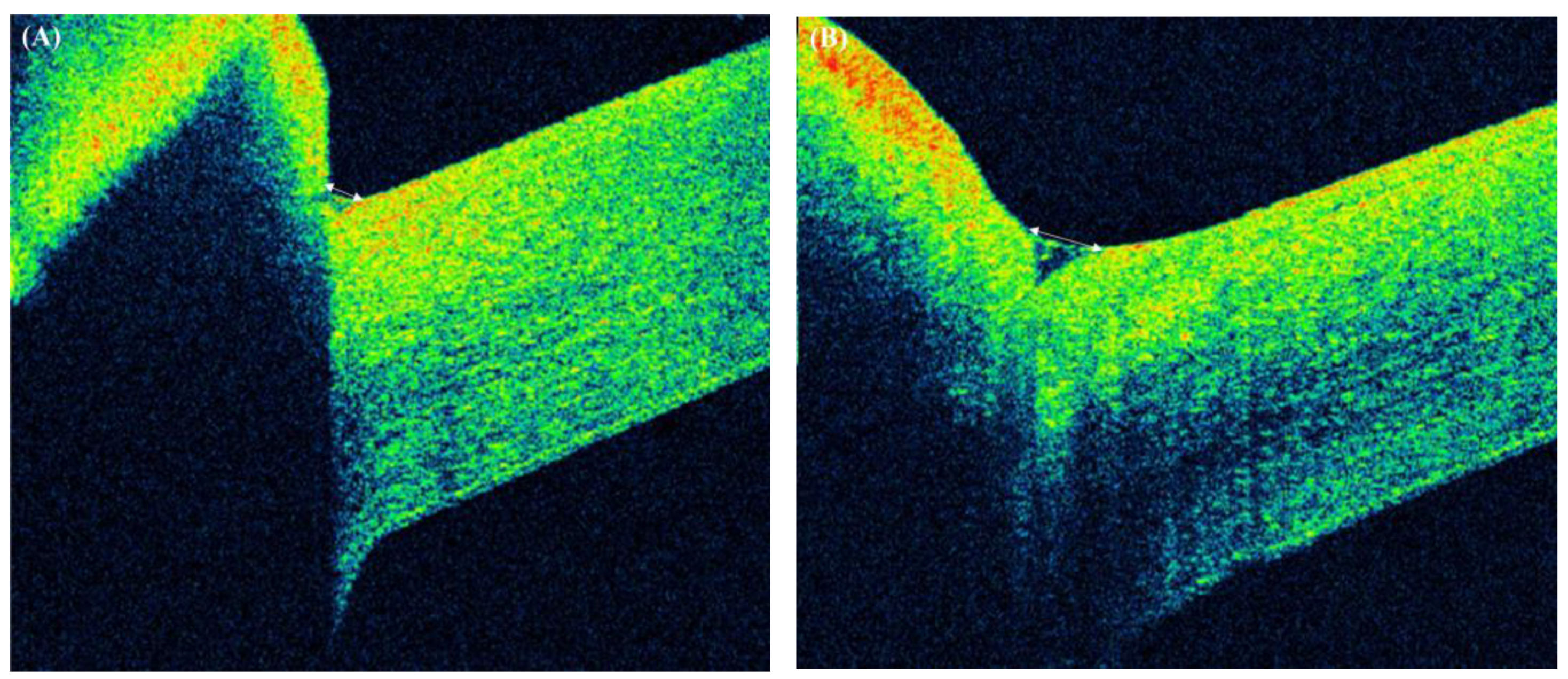
| Before | 1 Month after BTA Injection | p Value | |
|---|---|---|---|
| OSDI score | 62.22 ± 21.30 | 47.98 ± 17.23 | <0.001 |
| Tear osmolarity (mOsm/L) * | 320.82 ± 24.66 | 302.75 ± 22.33 | <0.001 |
| Schirmer test (mm) | 6.11 ± 2.51 | 7.18 ± 4.13 | 0.100 |
| TBUT (sec) | 3.07 ± 0.97 | 3.30 ± 1.06 | 0.229 |
| TMH (mm) | 82.25 ± 40.50 | 138.02 ± 66.62 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.W.; Yeom, D.J.; Jang, S.Y. Botulinum Toxin A Injection for the Treatment of Intractable Dry Eye Disease. Medicina 2021, 57, 247. https://doi.org/10.3390/medicina57030247
Choi EW, Yeom DJ, Jang SY. Botulinum Toxin A Injection for the Treatment of Intractable Dry Eye Disease. Medicina. 2021; 57(3):247. https://doi.org/10.3390/medicina57030247
Chicago/Turabian StyleChoi, Eun Woo, Dong Ju Yeom, and Sun Young Jang. 2021. "Botulinum Toxin A Injection for the Treatment of Intractable Dry Eye Disease" Medicina 57, no. 3: 247. https://doi.org/10.3390/medicina57030247
APA StyleChoi, E. W., Yeom, D. J., & Jang, S. Y. (2021). Botulinum Toxin A Injection for the Treatment of Intractable Dry Eye Disease. Medicina, 57(3), 247. https://doi.org/10.3390/medicina57030247





