The Prevalence of Ureaplasma Urealyticum and Mycoplasma Hominis Infections in Infertile Patients in the Northeast Region of Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Sample Collection and Processing
2.3. Ethical Approval
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greil, A.L.; Slauson-Blevins, K.; McQuillan, J. The experience of infertility: A review of recent literature. Sociol. Health Illn. 2010, 32, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Group ECW. Diagnosis and management of the infertile couple: Missing information. Hum. Reprod. Update 2004, 10, 295–307. [Google Scholar] [CrossRef]
- Thonneau, P.; Marchand, S.; Tallec, A.; Ferial, M.-L.; Ducot, B.; Lansac, J.; Lopes, P.; Tabaste, J.-M.; Spira, A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989)*. Hum. Reprod. 1991, 6, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.; Gerber-Schäfer, C.; Clad, A.; Wilhelm, C.; Breckwoldt, M. Seminal tract infections: Impact on male fertility and treatment options. Hum. Reprod. Update 1998, 4, 891–903. [Google Scholar] [CrossRef]
- Ness, R.B.; Goodman, M.T.; Shen, C.; Brunham, R.C. Serologic Evidence of Past Infection with Chlamydia trachomatis in Relation to Ovarian Cancer. J. Infect. Dis. 2003, 187, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Goy, G.; Jaton, K.; Osterheld, M.-C.; Blumer, S.; Borel, N.; Vial, Y.; Hohlfeld, P.; Pospischil, A.; Greub, G. Role of Chlamydia trachomatis in miscarriage. Emerg. Infect. Dis. 2011, 17, 1630–1635. [Google Scholar] [CrossRef]
- Shanmughapriya, S.; SenthilKumar, G.; Vinodhini, K.; Das, B.C.; Vasanthi, N.; Natarajaseenivasan, K. Viral and bacterial aetiologies of epithelial ovarian cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2311–2317. [Google Scholar] [CrossRef]
- Leli, C.; Mencacci, A.; Bombaci, J.; D’Alò, F.; Farinelli, S.; Vitali, M.; Montagna, P.; Bietolini, C.; Meucci, M.; Perito, S.; et al. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in a population of Italian and immigrant outpatients. InfezMed 2012, 20, 82–87. [Google Scholar]
- Zhang, N.; Wang, R.; Li, X.; Liu, X.; Tang, Z.; Liu, Y. Are Ureaplasma spp. a Cause of Nongonococcal Urethritis? A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e113771. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, C.; Cappello, N.; Latino, M.A.; Masuelli, G.; Marini, S.; Benedetto, C. Vaginal and endocervical microorganisms in symptomatic and asymptomatic non-pregnant females: Risk factors and rates of occurrence. Clin. Microbiol. Infect. 2009, 15, 670–679. [Google Scholar] [CrossRef][Green Version]
- Lanzafame, M.; Delama, A.; Emanuela, L.; Faggian, F.; Padovani, G.; Concia, E.; Vento, S. Prevalence and clinical significance of Ureaplasma urealyticum and Mycoplasma hominis in the lower genital tract of HIV-1-infected women. InfezMed 2007, 14, 213–215. [Google Scholar]
- Stellrecht, K.A.; Woron, A.M.; Mishrik, N.G.; Venezia, R.A. Comparison of multiplex PCR assay with culture for detection of genital mycoplasmas. J. Clin. Microbiol. 2004, 42, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.; Valentin, N.; Bogdan, D.; Carmen, C.M.; Coralia, B.; Demetra, S. Antibiotic susceptibility profiles of Mycoplasma hominis and Ureaplasma urealyticum isolated during a population-based study concerning women infertility in northeast romania. Braz. J. Microbiol. 2011, 42, 256–260. [Google Scholar] [CrossRef]
- Taylor-Robinson, D. Mollicutes in vaginal microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Res. Microbiol. 2017, 168, 875–881. [Google Scholar] [CrossRef]
- Baka, S.; Kouskouni, E.; Antonopoulou, S.; Sioutis, D.; Papakonstantinou, M.; Hassiakos, D.; Logothetis, E.; Liapis, A. Prevalence of Ureaplasma urealyticum and Mycoplasma hominis in women with chronic urinary symptoms. Urology 2009, 74, 62–66. [Google Scholar] [CrossRef]
- Egawa, T.; Morioka, I.; Morisawa, T.; Yokoyama, N.; Nakao, H.; Ohashi, M.; Matsuo, M. Ureaplasma urealyticum and Mycoplasma hominis presence in umbilical cord is associated with pathogenesis of funisitis. Kobe J. Med. Sci. 2007, 53, 241–249. [Google Scholar]
- Goldenberg, R.L.; Andrews, W.W.; Goepfert, A.R.; Faye-Petersen, O.; Cliver, S.P.; Carlo, W.A.; Hauth, J.C. The Alabama Preterm Birth Study: Umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J. Obs. Gynecol. 2008, 198, 43.e1–43.e435. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Xin, N.; Tong, X.-N.; Wang, J.-Y.; Liu, Z.-W. Prevalence and antibiotic susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Xi’an, China. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1941–1947. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, W.; Cui, D.; Nie, Z.; Ding, B.; Cheng, J.; Mei, C.-z. Examination of Ureaplasma urealyticum and Mycoplasma hominis in 4082 Chinese patients. Braz. J. Med. Biol. Res. 2021, 54, e10099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Y.; Li, R.-H.; Zheng, L.-Q.; Shang, X.-H. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in female outpatients, 2009–2013. J. Microbiol. Immunol. Infect. 2014, 49, 359–362. [Google Scholar] [CrossRef]
- Huang, C.; Long, X.; Jing, S.; Fan, L.; Xu, K.; Wang, S.; Zhu, W. Ureaplasma urealyticum and Mycoplasma hominis infections and semen quality in 19,098 infertile men in China. World J. Urol. 2016, 34, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, J.; Ling, Y.; Dong, C.; Wu, T.; Yu, X.; Hou, Y.; Dong, L.; Cheng, X. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Chinese women with genital infectious diseases. Indian J. Derm. Venereol. Leprol. 2012, 78, 406–407. [Google Scholar]
- Lee, J.S.; Kim, K.T.; Lee, H.S.; Yang, K.M.; Seo, J.T.; Choe, J.H. Concordance of Ureaplasma urealyticum and Mycoplasma hominis in infertile couples: Impact on semen parameters. Urology 2013, 81, 1219–1224. [Google Scholar] [CrossRef]
- Salmeri, M.; Valenti, D.; Vignera, S.; Bellanca, S.; Morello, A.; Toscano, M.; Mastrojeni, S.; Calogero, A.E. Prevalence of Ureaplasma urealyticum and Mycoplasma hominis infection in unselected infertile men. J. Chemother. 2012, 24, 81–86. [Google Scholar] [CrossRef]
- Moridi, K.; Hemmaty, M.; Azimian, A.; Fallah, M.H.; Khaneghahi Abyaneh, H.; Ghazvini, K. Epidemiology of genital infections caused by Mycoplasma hominis, M. genitalium and Ureaplasma urealyticum in Iran; a systematic review and meta-analysis study (2000–2019). BMC Public Health 2020, 20, 1020. [Google Scholar] [CrossRef]
- Daniele, M.; Ruiz, F.; Pascual, L.; Barberis, L. Ureaplasma urealyticum and Mycoplasma hominis Sensitivity to Bacteriocins Produced by Two Lactobacilli Strains. Curr. Microbiol. 2011, 63, 360. [Google Scholar] [CrossRef]
- Michou, I.V.; Constantoulakis, P.; Makarounis, K.; Georgoulias, G.; Kapetanios, V.; Tsilivakos, V. Molecular investigation of menstrual tissue for the presence of Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis collected by women with a history of infertility. J. Obs. Gynaecol. Res. 2014, 40, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S.; Kligman, I.; Grifo, J.A.; Rosenwaks, Z. Ureaplasma urealyticum and Mycoplasma hominis detected by the polymerase chain reaction in the cervices of women undergoingin vitro fertilization: Prevalence and consequences. J. Assist. Reprod. Genet. 1995, 12, 610–614. [Google Scholar] [CrossRef]
- Aydin, Y.; Atis, A.; Ocer, F.; Isenkul, R. Association of cervical infection of Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis with peritoneum colonisation in pregnancy. J. Obs. Gynaecol. 2010, 30, 809–812. [Google Scholar] [CrossRef]
- Garzon, S.; Laganà, A.S.; Casarin, J.; Raffaelli, R.; Cromi, A.; Sturla, D.; Franchi, M.; Ghezzi, F. An update on treatment options for interstitial cystitis. Prz. Menopauzalny 2020, 19, 35–43. [Google Scholar] [CrossRef]
- Patnaik, S.S.; Laganà, A.S.; Vitale, S.G.; Butticè, S.; Noventa, M.; Gizzo, S.; Valenti, G.; Rapisarda, A.M.C.; La Rosa, V.L.; Magno, C. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch. Gynecol. Obstet. 2017, 295, 1341–1359. [Google Scholar] [CrossRef] [PubMed]
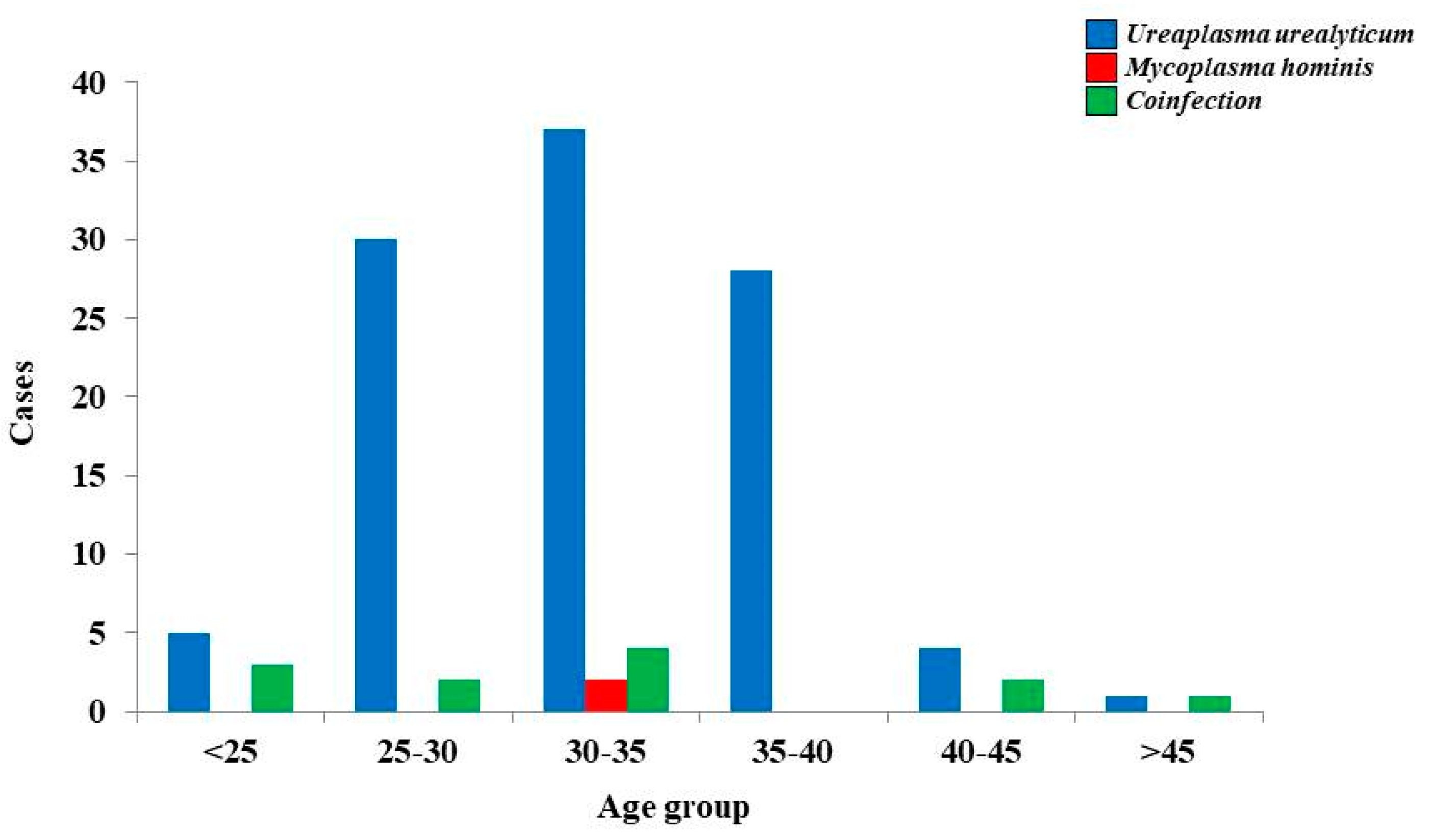
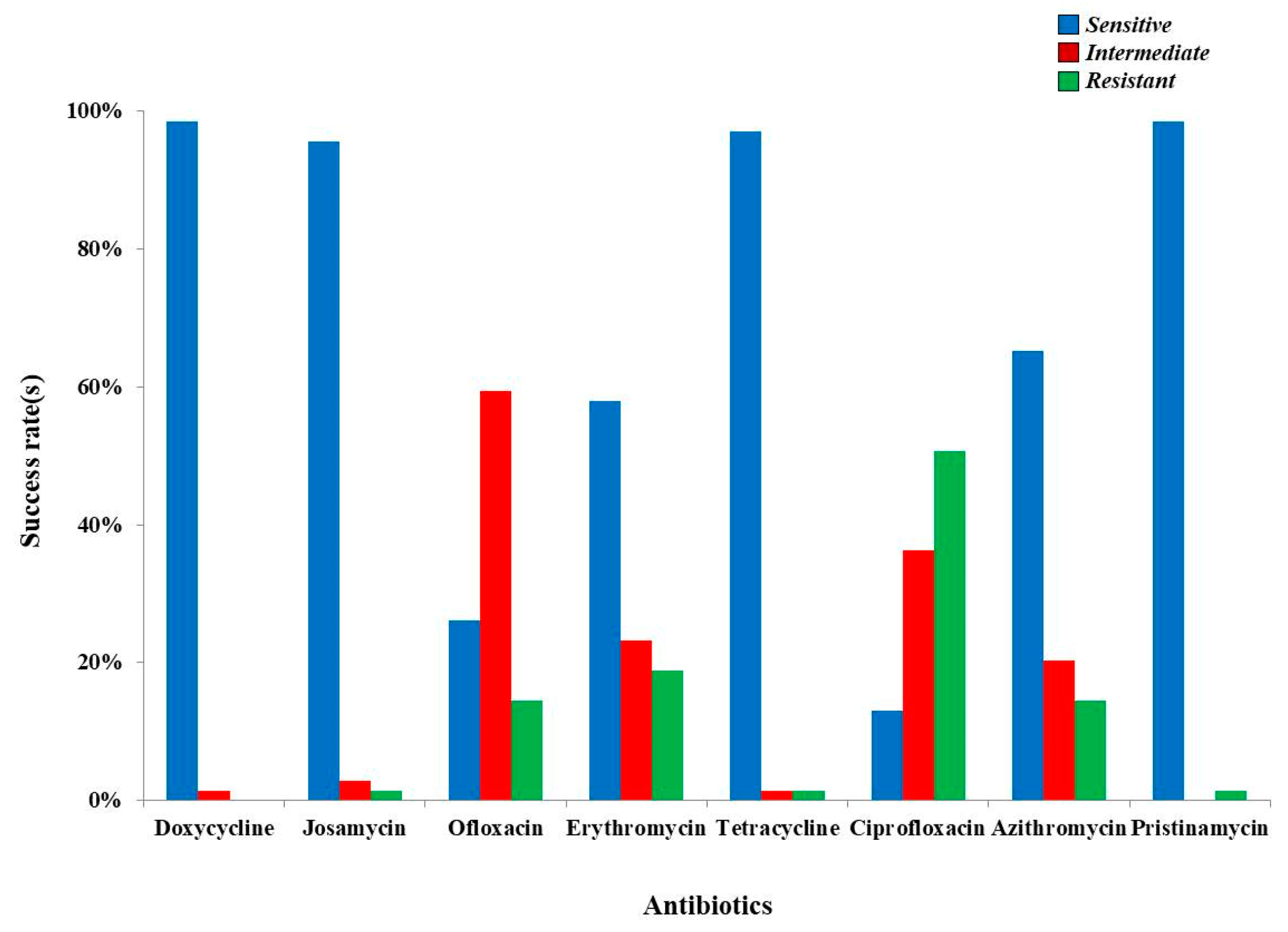
| Antibiotics | UU (Positive, n = 55) and MH (Positive, n = 2) | |||||
|---|---|---|---|---|---|---|
| Cumulative Results | Results based on the Age | |||||
| 20–30 (n = 19) | 30–40 (n = 33) | 40–50 (n = 4) | >51 (n = 1) | |||
| Doxycycline | S = 54; 94.73% | S = 2; 3.50% | S = 18 | S = 33 | S = 4 | S = 1 |
| I = 1; 1.75% | I = 0; 0.00% | I = 1 | I = 0 | I = 0 | I = 0 | |
| R = 0; 0.00% | R = 0; 0.00% | R = 0 | R = 0 | R = 0 | R = 0 | |
| Josamycin | S =55; 96.49% | S = 2; 3.50% | S= 19 | S = 33 | S = 4 | S = 1 |
| I = 0; 0.00% | I = 0; 0.00% | I = 0 | I = 0 | I = 0 | I = 0 | |
| R = 0; 0.00% | R = 0; 0.00% | R = 0 | R = 0 | R = 0 | R = 0 | |
| Ofloxacin | S = 11; 19.29% | S = 2; 3.50% | S = 4 | S = 9 | S = 0 | S = 0 |
| I = 35; 61.40% | I = 0; 0.00% | I = 12 | I = 19 | I = 3 | I = 1 | |
| R = 9; 15.78%S | R= 0; 0.00% | R = 3 | R = 5 | R = 1 | R = 0 | |
| Erythromycin | S = 40; 70.17% | S = 0; 0.00% | S = 13 | S = 24 | S = 2 | S = 1 |
| I = 14; 24.56% | I = 0; 0.00% | I = 6 | I = 6 | I = 2 | I = 0 | |
| R = 1; 1.75% | R = 2; 3.50% | R = 0 | R = 3 | R = 0 | R = 0 | |
| Tetracycline | S = 53; 92.98% | S = 2; 3.50% | S = 17 | S = 33 | S = 4 | S = 1 |
| I = 1; 1.75% | I = 0; 0.00% | I = 1 | I = 0 | R = 0 | I = 0 | |
| R = 1; 1.75% | R = 0; 0.00% | R = 1 | R = 0 | I = 0 | R = 0 | |
| Ciprofloxacin | S = 5; 8.77% | S = 2; 3.50% | S = 1 | S = 6 | S = 0 | S = 0 |
| I = 21; 31.84% | I = 0; 0.00% | I = 7 | I = 13 | I = 0 | I = 1 | |
| R = 29; 50.87% | R = 0; 0.00% | R = 11 | R = 14 | R = 4 | R = 0 | |
| Azithromycin | S = 45; 78.94% | S = 0; 0.00% | S = 17 | S = 25 | S = 2 | S = 1 |
| I = 10; 17.54% | I = 0; 0.00% | I = 2 | I = 6 | I = 2 | I = 0 | |
| R = 0; 0.00% | R = 2; 3.50% | R = 0 | R = 2 | R = 0 | R = 0 | |
| Pristinamycin | S = 55; 96.49% | S = 2; 3.50% | S = 19 | S = 33 | S = 4 | S = 1 |
| I = 0; 0.00% | I = 0; 0.00% | I = 0 | I = 0 | I = 0 | I = 0 | |
| R = 0; 0.00% | R = 0; 0.00% | R = 0 | R = 0 | R = 0 | R = 0 | |
| Coinfection (n = 12) | ||||||
| 20–30 (n = 5) | 30–40 (n = 4) | 40–50 (n = 3) | >51 (n = 0) | |||
| S = 5 | S = 4 | S = 3 | S = 0 | |||
| I = 0 | I = 0 | I = 0 | I = 0 | |||
| R = 0 | R = 0 | R = 0 | R = 0 | |||
| S = 4 | S = 3 | S = 2 | S = 0 | |||
| I = 1 | I = 1 | I = 0 | I = 0 | |||
| R = 0 | R = 0 | R = 1 | R = 0 | |||
| S = 2 | S = 2 | S = 1 | S = 0 | |||
| I = 3 | I = 1 | I = 2 | I = 0 | |||
| R = 0 | R = 1 | R = 0 | R = 0 | |||
| S = 0 | S = 0 | S = 0 | S = 0 | |||
| I = 0 | I = 1 | I = 1 | I = 0 | |||
| R = 5 | R = 3 | R = 2 | R = 0 | |||
| S = 5 | S = 4 | S = 3 | S = 0 | |||
| I = 0 | I = 0 | I = 0 | I = 0 | |||
| R = 0 | R = 0 | R = 0 | R = 0 | |||
| S = 0 | S = 1 | S = 1 | S = 0 | |||
| I = 3 | I = 1 | I = 0 | I = 0 | |||
| R = 2 | R = 2 | R = 2 | R = 0 | |||
| S = 0 | S = 0 | S = 0 | S = 0 | |||
| I = 2 | I = 1 | I = 1 | I = 0 | |||
| R = 3 | R = 3 | R = 2 | R = 0 | |||
| S = 5 | S = 4 | S = 3 | S = 0 | |||
| I = 0 | I = 0 | I = 0 | I = 0 | |||
| R = 0 | R = 0 | R = 0 | R = 0 | |||
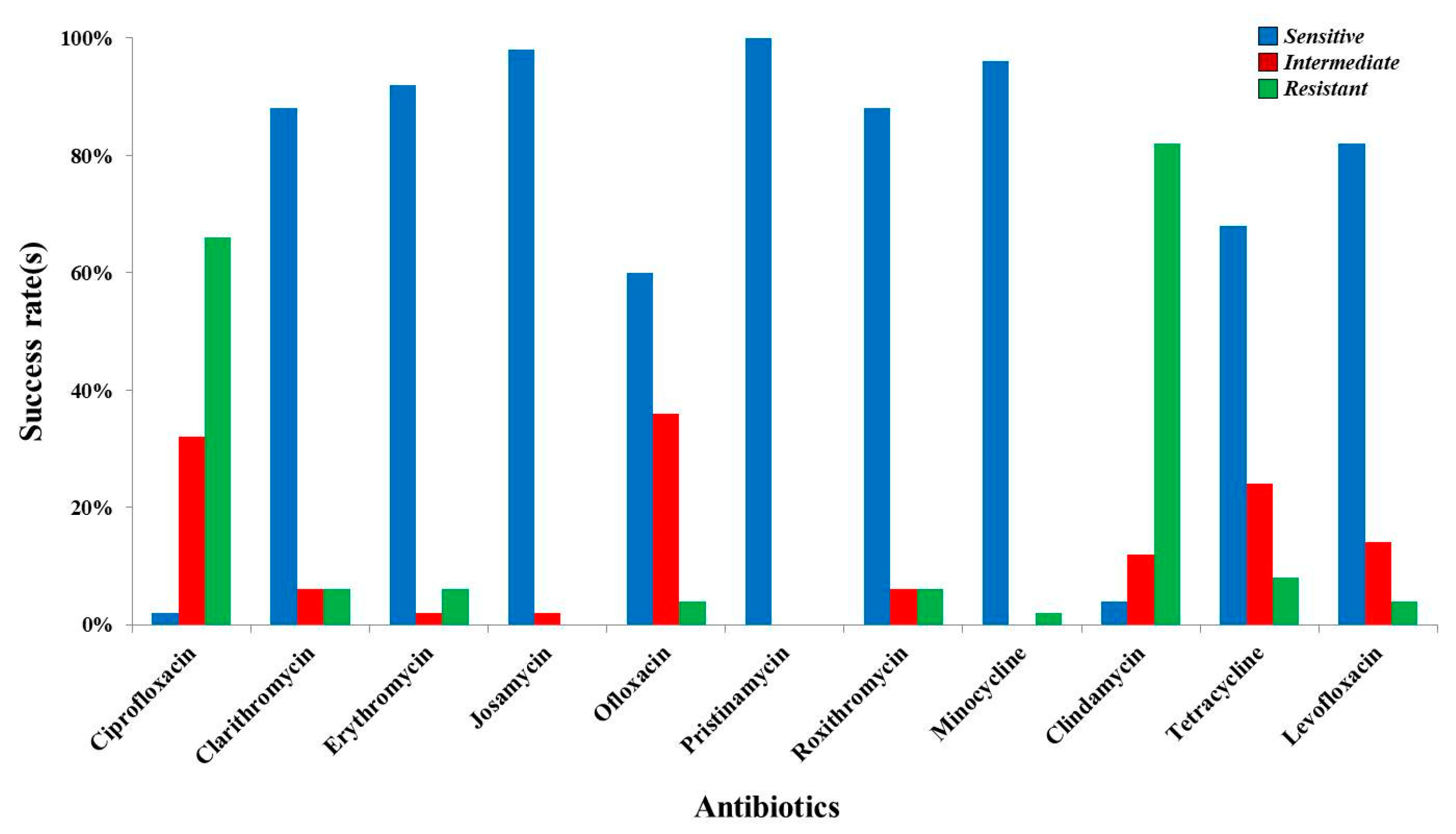
| Antibiotics | UU (Positive, n = 50) and MH (Positive, n = 0) | |||||
|---|---|---|---|---|---|---|
| Cumulative Results | Results based on the Age | |||||
| 20–30 (n = 16) | 30–40 (n = 33) | 40–50 (n = 1) | >51 (n = 0) | |||
| Ciprofloxacin | S = 1; 2.00% | S = 0; 0.00% | S = 0 | S = 1 | S = 0 | S = 0 |
| I = 16; 32.00% | I = 0; 0.00% | I = 6 | I = 10 | I = 0 | I = 0 | |
| R = 33; 66.00% | R = 0; 0.00% | R = 10 | R = 22 | R = 1 | R = 0 | |
| Clarithromycin | S = 44; 88.00% | S = 0; 0.00% | S =14 | S = 29 | S = 1 | S = 0 |
| I = 3; 6.00% | I = 0; 0.00% | I = 1 | I = 2 | I = 0 | I = 0 | |
| R = 3; 6.00% | R = 0; 0.00% | R = 1 | R = 2 | R = 0 | R = 0 | |
| Erythromycin | S = 46; 92.00% | S = 0; 0.00% | S = 15 | S = 30 | S = 1 | S = 0 |
| I = 1; 2.00% | I = 0; 0.00% | I = 0 | I = 2 | I = 0 | I = 0 | |
| R = 3; 6.00% | R = 0; 0.00% | R = 1 | R = 2 | R = 0 | R = 0 | |
| Josamycin | S = 49; 98.00% | S = 0; 0.00% | S = 16 | S = 32 | S = 1 | S = 0 |
| I = 1; 2.00% | I = 0; 0.00% | I = 0 | I = 1 | I = 0 | I = 0 | |
| R = 0; 0.00% | R = 0; 0.00% | R = 0 | R = 0 | R = 0 | R = 0 | |
| Ofloxacin | S = 30; 60.00% | S = 0; 0.00% | S = 10 | S = 19 | S = 1 | S = 0 |
| I = 18; 36.00% | I = 0; 0.00% | I = 6 | I = 12 | I = 0 | I = 0 | |
| R = 2; 4.00% | R = 0; 0.00% | R = 0 | R = 2 | R = 0 | R = 0 | |
| Pristinamycin | S = 50; 100% | S = 0; 0.00% | S = 16 | S = 33 | S = 1 | S = 0 |
| I = 0; 0.00% | I = 0; 0.00% | I = 0 | I = 0 | I = 0 | I = 0 | |
| R = 0; 0.00% | R = 0; 0.00% | R = 0 | R = 0 | R = 0 | R = 0 | |
| Roxithromycin | S= 44; 88.00% | S = 0; 0.00% | S = 14 | S = 29 | S = 1 | S = 0 |
| I = 3; 6.00% | I = 0; 0.00% | I = 1 | I = 2 | I = 0 | I = 0 | |
| R = 3; 6.00% | R = 0; 0.00% | R = 1 | R = 2 | R = 0 | R = 0 | |
| Minocycline | S = 48; 96.00% | S = 0; 0.00% | S = 15 | S = 32 | S = 1 | S = 0 |
| I = 0; 0.00% | I = 0; 0.00% | I = 0 | I = 0 | I = 0 | I = 0 | |
| R = 1; 2.00% | R = 0; 0.00% | R = 0 | R = 1 | R = 0 | R = 0 | |
| Clindamycin | S = 2; 4.00% | S = 0; 0.00% | S = 1 | S = 1 | S = 0 | S = 0 |
| I = 6; 12.00% | I = 0; 0.00% | I = 0 | R = 6 | I = 0 | I = 0 | |
| R = 41; 82.00% | R = 0; 0.00% | R = 15 | R = 25 | R = 1 | R = 0 | |
| Tetracycline | S = 34; 68.00% | S = 0; 0.00% | S = 11 | S = 23 | S = 0 | S = 0 |
| I = 12; 24.00% | I = 0; 0.00% | I = 4 | I = 7 | I = 1 | I = 0 | |
| R = 4; 8.00% | R = 0; 0.00% | R = 1 | R = 3 | R = 0 | R = 0 | |
| Levofloxacin | S = 41; 82.00% | S = 0; 0.00% | S = 14 | S = 26 | S = 1 | S = 0 |
| I = 7; 14.00% | I = 0; 0.00% | I = 2 | I = 5 | I = 0 | I = 0 | |
| R = 2; 4.00% | R = 0; 0.00% | R = 0 | R = 2 | R = 0 | R = 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroftei, B.; Ilie, O.-D.; Armeanu, T.; Anton, E.; Scripcariu, I.; Maftei, R. The Prevalence of Ureaplasma Urealyticum and Mycoplasma Hominis Infections in Infertile Patients in the Northeast Region of Romania. Medicina 2021, 57, 211. https://doi.org/10.3390/medicina57030211
Doroftei B, Ilie O-D, Armeanu T, Anton E, Scripcariu I, Maftei R. The Prevalence of Ureaplasma Urealyticum and Mycoplasma Hominis Infections in Infertile Patients in the Northeast Region of Romania. Medicina. 2021; 57(3):211. https://doi.org/10.3390/medicina57030211
Chicago/Turabian StyleDoroftei, Bogdan, Ovidiu-Dumitru Ilie, Theodora Armeanu, Emil Anton, Ioana Scripcariu, and Radu Maftei. 2021. "The Prevalence of Ureaplasma Urealyticum and Mycoplasma Hominis Infections in Infertile Patients in the Northeast Region of Romania" Medicina 57, no. 3: 211. https://doi.org/10.3390/medicina57030211
APA StyleDoroftei, B., Ilie, O.-D., Armeanu, T., Anton, E., Scripcariu, I., & Maftei, R. (2021). The Prevalence of Ureaplasma Urealyticum and Mycoplasma Hominis Infections in Infertile Patients in the Northeast Region of Romania. Medicina, 57(3), 211. https://doi.org/10.3390/medicina57030211








