Effects of Antiviral Therapy and Glucocorticoid Therapy on Fever Duration in Pediatric Patients with Influenza
Abstract
:1. Introduction
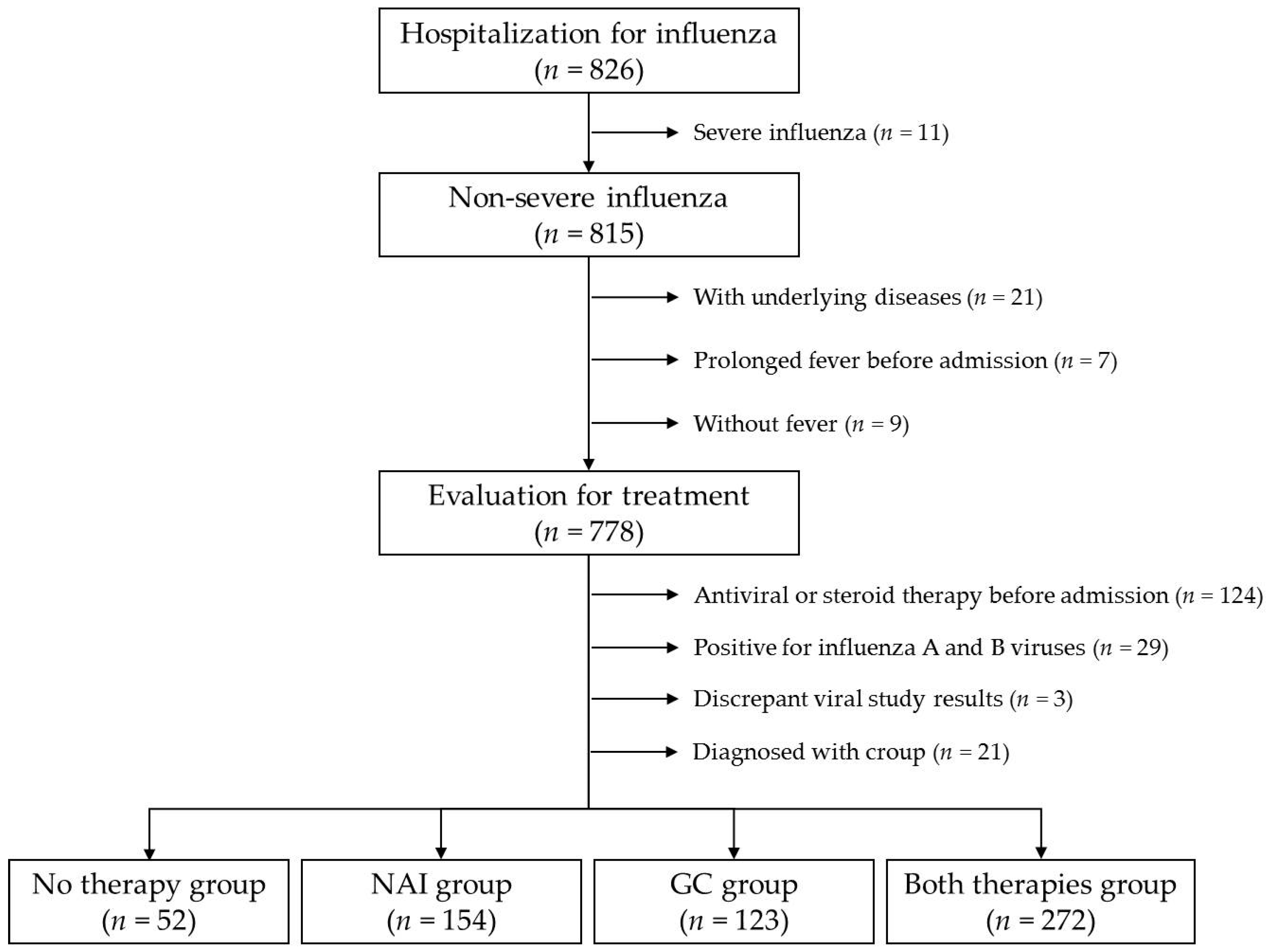
2. Materials and Methods
2.1. Subject and Data Analysis
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Four Treatment Groups
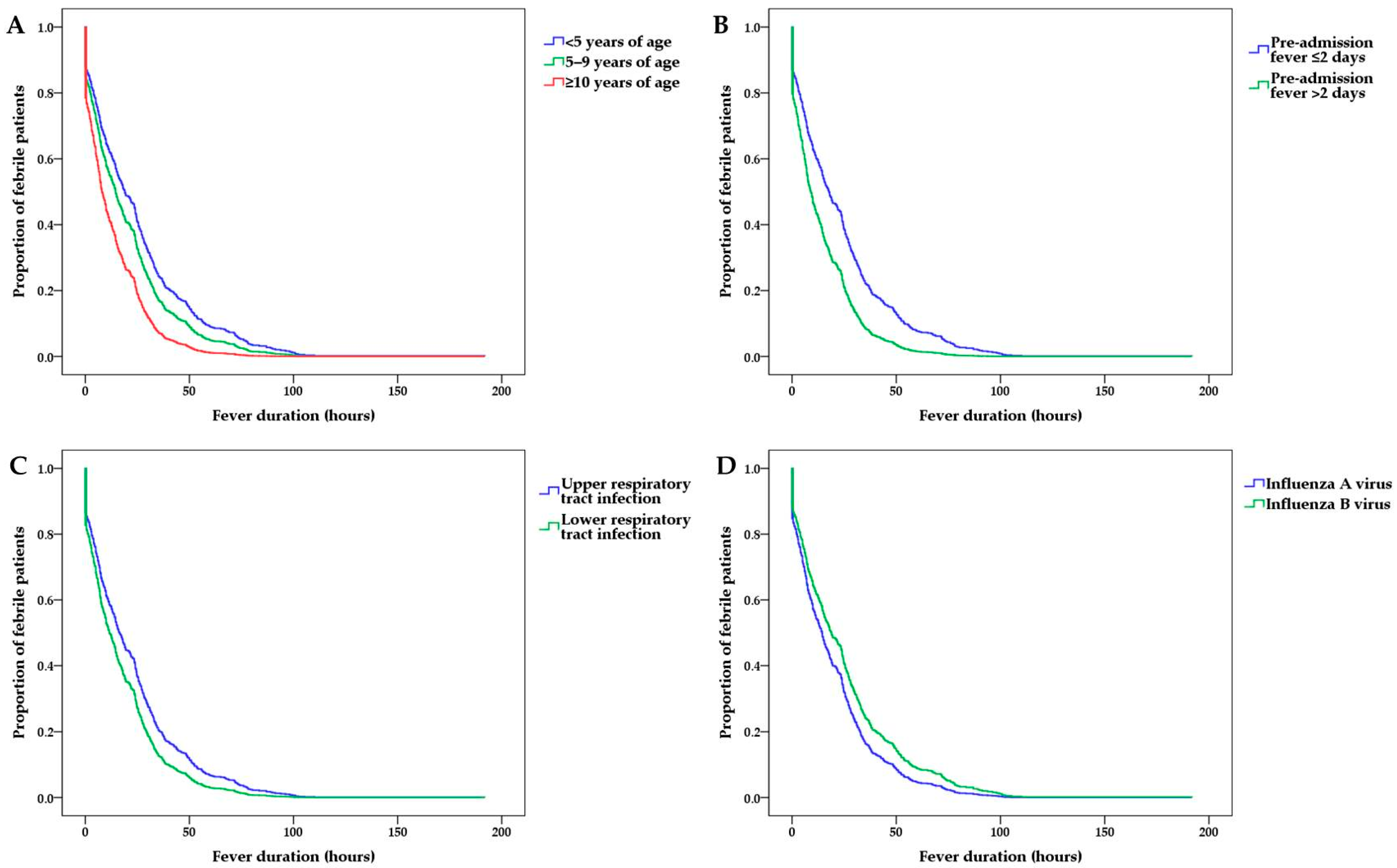
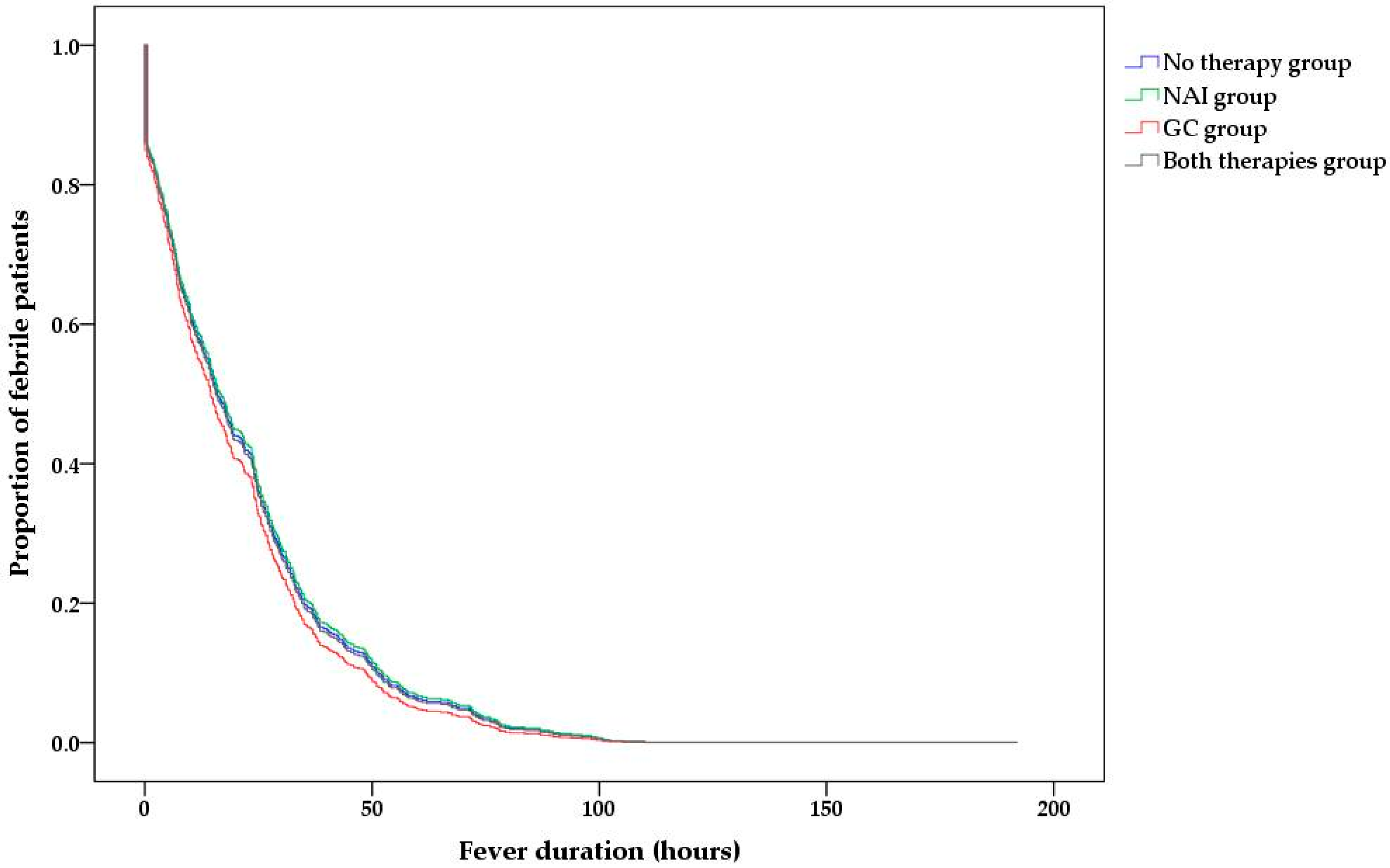
3.2. Factors Associated with the Post-Admission Fever Duration
3.3. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Influenza (Seasonal) Fact Sheet. Available online: https://who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 1 November 2021).
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.; Lee, N.; Chan, P.K.; Beigel, J.H. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antivir. Res. 2018, 150, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Rodrigo, C.; Leonardi-Bee, J.; Nguyen-Van-Tam, J.; Lim, W.S. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst. Rev. 2019, 2, CD010406. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, S.G.; Gu, L.; Zhang, Y.; Yan, X.X.; Liang, Z.A.; Zhang, W.; Jia, H.Y.; Chen, W.; Liu, M.; et al. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influ. Other Respir. Viruses 2017, 11, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Rhim, J.W.; Kang, J.H.; Lee, K.Y. Clinical features and outcomes of influenza by virus type/subtype/lineage in pediatric patients. Transl. Pediatr. 2021, 10, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Thomas, P.G. New fronts emerge in the influenza cytokine storm. Semin. Immunopathol. 2017, 39, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Gates, A.; Gates, M.; Vandermeer, B.; Johnson, C.; Hartling, L.; Johnson, D.W.; Klassen, T.P. Glucocorticoids for croup in children. Cochrane Database Syst. Rev. 2018, 8, CD001955. [Google Scholar] [CrossRef]
- Malosh, R.E.; Martin, E.T.; Heikkinen, T.; Brooks, W.A.; Whitley, R.J.; Monto, A.S. Efficacy and safety of oseltamivir in children: Systematic review and individual patient data meta-analysis of randomized controlled trials. Clin. Infect. Dis. 2018, 66, 1492–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, M.; Calvo, C.; Mendez-Echevarria, A.; de Jose, M.I.; Santos, M.; Carrasco, J.; Tovizi, M.; Guillén, S.; de Blas, A.; Llorente, M.; et al. Oseltamivir treatment for influenza in hospitalized children without underlying diseases. Pediatr. Infect. Dis. J. 2013, 32, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Bianchini, S.; Baggi, E.; Esposito, S. No evidence for the effectiveness of systemic corticosteroids in acute pharyngitis, community-acquired pneumonia and acute otitis media. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Do, Y.K. Prescription of systemic steroids for acute respiratory infections in Korean outpatient settings: Overall patterns and effects of the prescription appropriateness evaluation policy. J. Prev. Med. Public Health 2020, 53, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.J.; Dvorin, E.; Kesselheim, A.S. Prescribing systemic steroids for acute respiratory tract infections in United States outpatient settings: A nationwide population-based cohort study. PLoS Med. 2020, 17, e1003058. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.F.; Fung, C.P.; Perng, D.W.; Wang, F.D. Effects of corticosteroid and neuraminidase inhibitors on survival in patients with respiratory distress induced by influenza virus. J. Microbiol. Immunol. Infect. 2017, 50, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Loeches, I.; Lisboa, T.; Rhodes, A.; Moreno, R.P.; Silva, E.; Sprung, C.; Chiche, J.D.; Barahona, D.; Villabon, M.; Balasini, C.; et al. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med. 2011, 37, 272–283. [Google Scholar] [CrossRef]
- Tsai, M.J.; Yang, K.Y.; Chan, M.C.; Kao, K.C.; Wang, H.C.; Perng, W.C.; Wu, C.L.; Liang, S.J.; Fang, W.F.; Tsai, J.R.; et al. Impact of corticosteroid treatment on clinical outcomes of influenza-associated ARDS: A nationwide multicenter study. Ann. Intensive Care 2020, 10, 26. [Google Scholar] [CrossRef]
- Brun-Buisson, C.; Richard, J.C.; Mercat, A.; Thiebaut, A.C.; Brochard, L.; REVA-SRLF A/H1N1v 2009 Registry Group. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2011, 183, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, S.B.; Yun, S.C.; Choi, W.I.; Ahn, J.J.; Lee, Y.J.; Lee, H.B.; Lim, C.M.; Koh, Y.; Korean Society of Critical Care Medicine H1N1 Collaborative. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: Analytic strategy using propensity scores. Am. J. Respir. Crit. Care Med. 2011, 183, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Moreno, G.; Rodriguez, A.; Reyes, L.F.; Gomez, J.; Sole-Violan, J.; Diaz, E.; Bodí, M.; Trefler, S.; Guardiola, J.; Yébenes, J.C.; et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: A propensity score matching study. Intensive Care Med. 2018, 44, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.W.; Pinto, R.; Long, J.; Lamontagne, F.; Adhikari, N.K.; Kumar, A.; Marshall, J.C.; Cook, D.J.; Jouvet, P.; Ferguson, N.D.; et al. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit. Care 2016, 20, 75. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Ma, H.; An, X.; Su, Y.; Chen, J.; Lian, Z.; Zhao, J.H.; Zhu, B.P.; Fontaine, R.E.; Feng, Z.; et al. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin. Infect. Dis. 2011, 53, 326–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, J.P.; Baker, A.J. Influenza-associated neurological complications. Neurocrit. Care 2013, 18, 118–130. [Google Scholar] [CrossRef]
- Frankl, S.; Coffin, S.E.; Harrison, J.B.; Swami, S.K.; McGuire, J.L. Influenza-associated neurologic complications in hospitalized children. J. Pediatr. 2021, 239, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.J.; Park, J.Y.; Choi, J.S.; Choi, S.R.; Kim, D.; Lee, J.H.; Woo, Y.J.; Lee, J.; Kim, Y.J. Influenza-associated neurologic complications in hospitalized pediatric patients: A multicenter retrospective study in Republic of Korea. Pediatr. Infect. Dis. J. 2021, 40, e466–e471. [Google Scholar] [CrossRef] [PubMed]
- Hatachi, T.; Michihata, N.; Takeuchi, M.; Matsui, H.; Fushimi, K.; Yasunaga, H. Early steroid pulse therapy among children with influenza virus-associated encephalopathy. J. Intensive Care 2020, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Vergu, E.; Ferguson, N.M.; Lemaitre, M.; Cauchemez, S.; Leach, S.; Valleron, A.J. Time lines of infection and disease in human influenza: A review of volunteer challenge studies. Am. J. Epidemiol. 2008, 167, 775–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KOrean Statistical Information Service. Available online: https://kosis.kr/index/index.do (accessed on 1 November 2021).
- Daoud, A.; Laktineh, A.; Macrander, C.; Mushtaq, A.; Soubani, A.O. Pulmonary complications of influenza infection: A targeted narrative review. Postgrad. Med. 2019, 131, 299–308. [Google Scholar] [CrossRef] [PubMed]
| Factor | No Therapy Group (n = 52) | NAI Group (n = 154) | GC Group (n = 123) | Both Therapies Group (n = 272) | p-Value |
|---|---|---|---|---|---|
| Male sex | 28 (53.8) | 77 (50.0) | 70 (56.9) | 149 (54.8) | 0.688 |
| Age, years, median (IQR) | 4 (1–7) | 5 (2–9) | 3 (1–6) | 4 (2–7) | 0.008 |
| Influenza virus subtype | 0.455 | ||||
| Influenza A virus | 33 (63.5) | 90 (58.4) | 83 (67.5) | 175 (64.3) | |
| Influenza B virus | 19 (36.5) | 64 (41.6) | 40 (32.5) | 97 (35.7) | |
| Diagnosis on admission | <0.001 | ||||
| URI | 50 (96.2) | 149 (96.8) | 91 (74.0) | 228 (83.8) | |
| LRI | 2 (3.8) | 5 (3.2) | 32 (26.0) | 44 (16.2) | |
| Final diagnosis | <0.001 | ||||
| URI | 50 (96.2) | 145 (94.2) | 90 (73.2) | 219 (80.5) | |
| LRI | 2 (3.8) | 9 (5.8) | 33 (26.8) | 53 (19.5) | |
| Symptoms | |||||
| Cough | 47 (90.4) | 136 (88.3) | 107 (87.0) | 247 (90.8) | 0.668 |
| Rhinorrhea | 42 (80.8) | 127 (82.5) | 98 (79.7) | 223 (82.0) | 0.934 |
| Sputum | 31 (59.6) | 95 (61.7) | 87 (70.7) | 189 (69.5) | 0.189 |
| Sore throat | 3 (5.8) | 24 (15.6) | 14 (11.4) | 24 (8.8) | 0.104 |
| Dyspnea | 0 (0.0) | 0 (0.0) | 1 (0.8) | 4 (1.5) | 0.380 |
| Vomiting | 14 (26.9) | 20 (13.0) | 17 (13.8) | 35 (12.9) | 0.059 |
| Abdominal pain | 6 (11.5) | 22 (14.3) | 9 (7.3) | 23 (8.5) | 0.172 |
| Diarrhea | 6 (11.5) | 18 (11.7) | 11 (8.9) | 19 (7.0) | 0.373 |
| Headache | 8 (15.4) | 24 (15.6) | 8 (6.5) | 18 (6.6) | 0.006 |
| Myalgia | 3 (5.8) | 15 (9.7) | 6 (4.9) | 17 (6.3) | 0.388 |
| Rash | 4 (7.7) | 2 (1.3) | 5 (4.1) | 2 (0.7) | 0.005 |
| Focal complications | |||||
| Acute otitis media | 7 (13.5) | 7 (4.5) | 4 (3.3) | 8 (2.9) | 0.007 |
| Sinusitis | 1 (1.9) | 2 (1.3) | 1 (0.8) | 2 (0.7) | 0.844 |
| Hospital days, median (IQR) | 4 (4–6) | 4 (4–5) | 4 (4–6) | 4 (4–5) | 0.974 |
| Fever duration, median (IQR) | |||||
| Before admission, days | 1 (1–3) | 1 (0–2) | 1 (1–2) | 1 (1–2) | 0.101 |
| After admission, hours | 13.8 (0.1–42.0) | 21.3 (7.2–33.9) | 11.0 (1.2–30.5) | 15.6 (4.6–31.5) | 0.066 |
| Overall, days | 3 (2–5) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.405 |
| Factor | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Female (vs. male) | 1.072 | 0.909–1.263 | 0.410 |
| Age, years | 0.954 | 0.934–0.975 | <0.001 |
| Pre-admission fever, days | 0.878 | 0.833–0.925 | <0.001 |
| Influenza A (vs. influenza B) | 0.782 | 0.656–0.932 | 0.006 |
| LRI on admission (vs. URI) | 0.626 | 0.489–0.801 | <0.001 |
| Type of administered treatment | |||
| No therapy | reference | ||
| NAI therapy | 1.022 | 0.744–1.404 | 0.892 |
| GC therapy | 0.951 | 0.682–1.328 | 0.769 |
| Both therapies | 0.990 | 0.732–1.340 | 0.949 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.Y.; Yang, E.A.; Rhim, J.-W.; Han, S.B. Effects of Antiviral Therapy and Glucocorticoid Therapy on Fever Duration in Pediatric Patients with Influenza. Medicina 2021, 57, 1385. https://doi.org/10.3390/medicina57121385
Han JY, Yang EA, Rhim J-W, Han SB. Effects of Antiviral Therapy and Glucocorticoid Therapy on Fever Duration in Pediatric Patients with Influenza. Medicina. 2021; 57(12):1385. https://doi.org/10.3390/medicina57121385
Chicago/Turabian StyleHan, Ji Yoon, Eun Ae Yang, Jung-Woo Rhim, and Seung Beom Han. 2021. "Effects of Antiviral Therapy and Glucocorticoid Therapy on Fever Duration in Pediatric Patients with Influenza" Medicina 57, no. 12: 1385. https://doi.org/10.3390/medicina57121385
APA StyleHan, J. Y., Yang, E. A., Rhim, J.-W., & Han, S. B. (2021). Effects of Antiviral Therapy and Glucocorticoid Therapy on Fever Duration in Pediatric Patients with Influenza. Medicina, 57(12), 1385. https://doi.org/10.3390/medicina57121385






