Combination Therapy with Rituximab, Tofacitinib and Pirfenidone in a Patient with Rapid Progressive Interstitial Lung Disease (RP-ILD) Due to MDA5 Antibody-Associated Dermatomyositis: A Case Report
Abstract
:1. Introduction
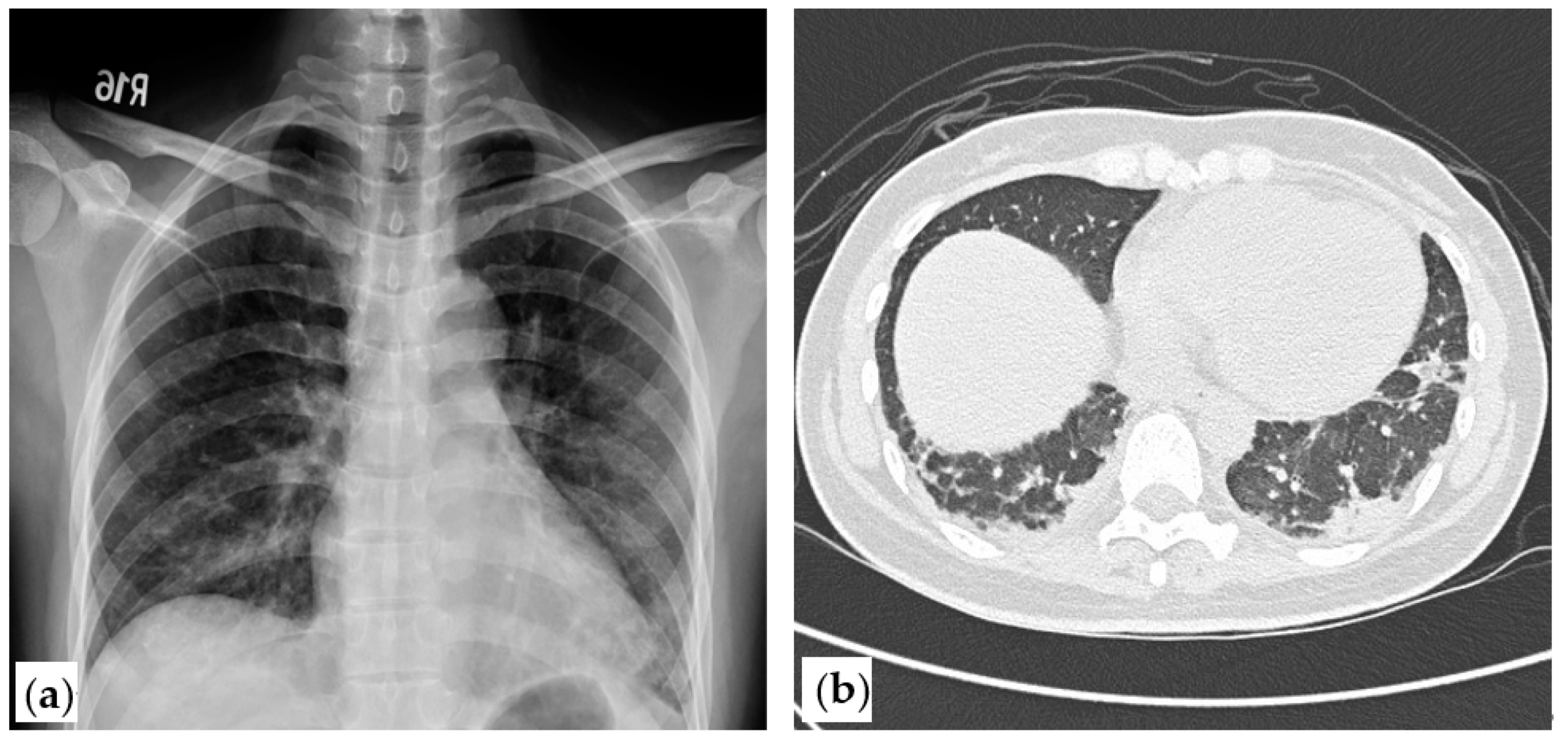
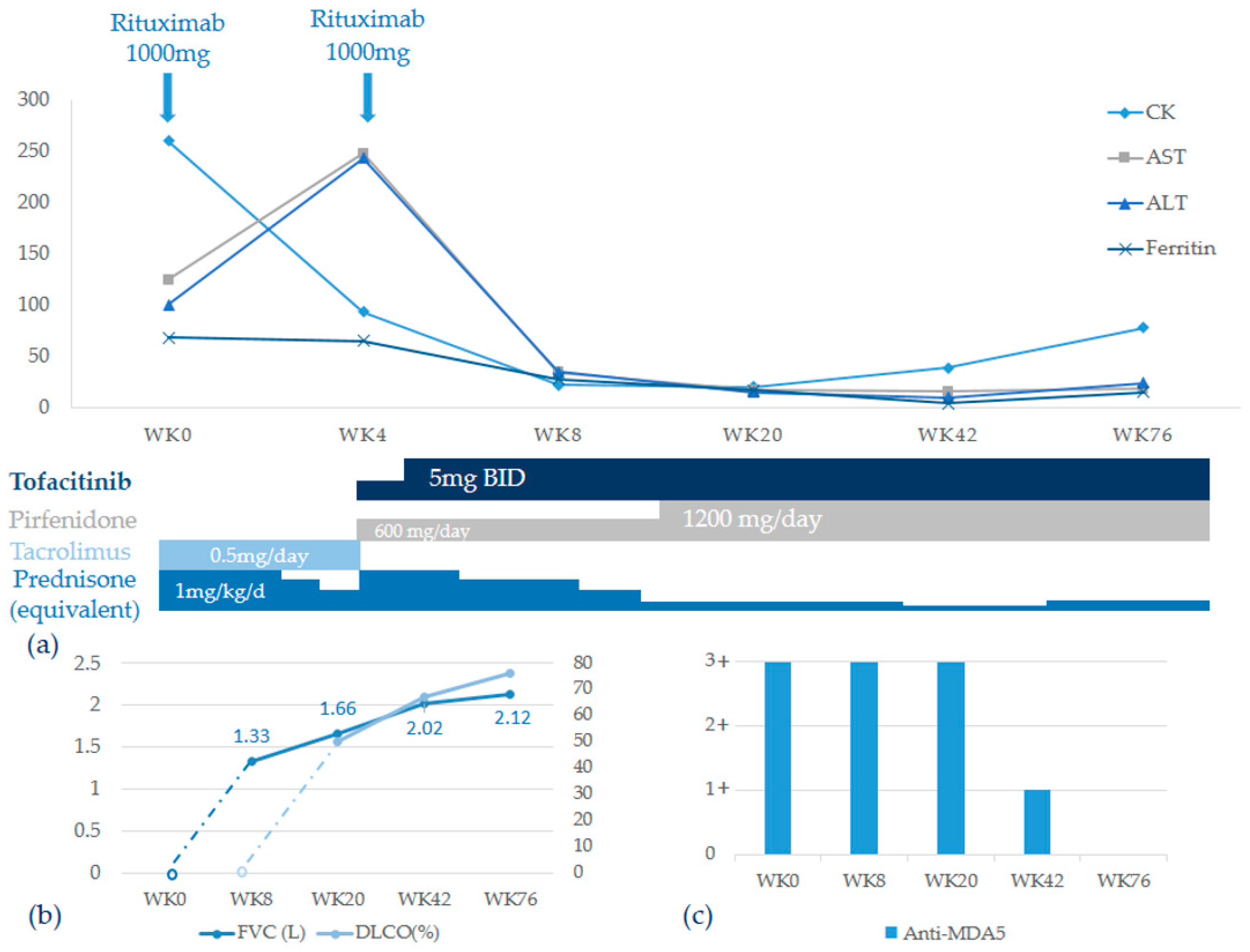
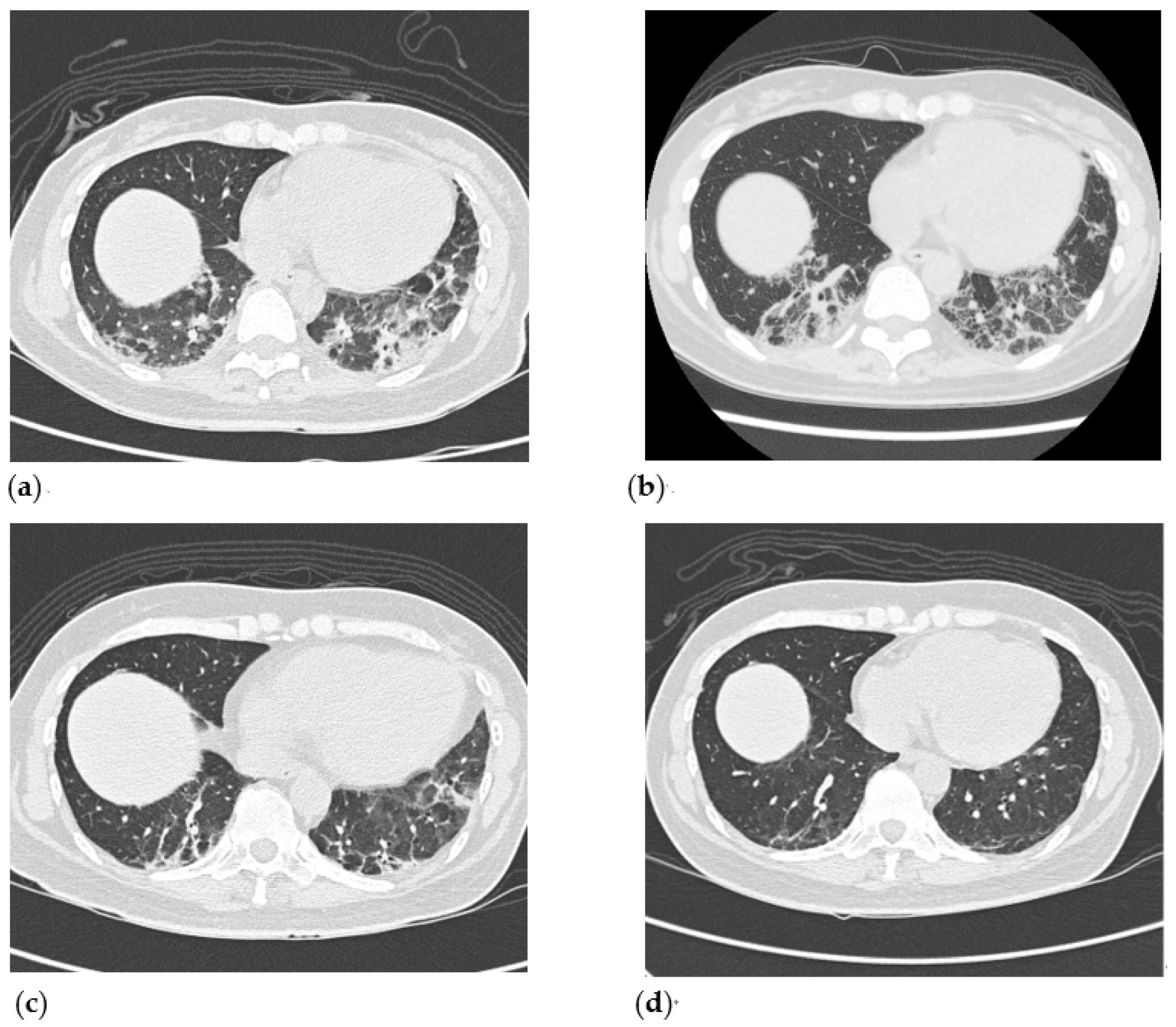
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hozumi, H.; Fujisawa, T.; Nakashima, R.; Johkoh, T.; Sumikawa, H.; Murakami, A.; Enomoto, N.; Inui, N.; Nakamura, Y.; Hosono, Y.; et al. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir. Med. 2016, 121, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, S.; Hirakata, M.; Kuwana, M.; Suwa, A.; Inada, S.; Mimori, T.; Nishikawa, T.; Oddis, C.V.; Ikeda, Y. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005, 52, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, D.J.B.; Vleugels, R.A. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: A concise review with an emphasis on distinctive clinical features. J. Am. Acad. Dermatol. 2018, 78, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Nakashima, R.; Hosono, Y.; Imura, Y.; Yagita, M.; Yoshifuji, H.; Hirata, S.; Nojima, T.; Sugiyama, E.; Hatta, K.; et al. Multicenter Prospective Study of the Efficacy and Safety of Combined Immunosuppressive Therapy with High-Dose Glucocorticoid, Tacrolimus, and Cyclophosphamide in Interstitial Lung Diseases Accompanied by Anti-Melanoma Differentiation-Associated Gene 5-Positive Dermatomyositis. Arthritis Rheumatol. 2020, 72, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Ye, S. Tofacitinib in Amyopathic Dermatomyositis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 381, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Guo, L.; Chen, Z.; Gu, L.; Sun, F.; Tan, X.; Chen, S.; Wang, X.; Ye, S. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci. Rep. 2016, 6, 33226. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Li, S.; Tian, X.; He, L.; Lu, X.; Wang, G. Anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis responds to rituximab therapy. Clin. Rheumatol. 2021, 40, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- So, H.; Wong, V.T.L.; Lao, V.W.N.; Pang, H.T.; Yip, R.M.L. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin. Rheumatol. 2018, 37, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Oddis, C.V.; Reed, A.M.; Aggarwal, R.; Rider, L.G.; Ascherman, D.P.; Levesque, M.C.; Barohn, R.J.; Feldman, B.M.; Harris-Love, M.O.; Koontz, D.C.; et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: A randomized, placebo-phase trial. Arthritis Rheum. 2013, 65, 314–324. [Google Scholar] [CrossRef]
- Kurasawa, K.; Arai, S.; Namiki, Y.; Tanaka, A.; Takamura, Y.; Owada, T.; Arima, M.; Maezawa, R. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology 2018, 57, 2114–2119. [Google Scholar] [CrossRef]
- Strand, V.; Kremer, J.; Wallenstein, G.; Kanik, K.S.; Connell, C.; Gruben, D.; Zwillich, S.H.; Fleischmann, R. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res. Ther. 2015, 17, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnolo, P.; Distler, O.; Ryerson, C.J.; Tzouvelekis, A.; Lee, J.S.; Bonella, F.; Bouros, D.; Hoffmann-Vold, A.-M.; Crestani, B.; Matteson, E.L. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann. Rheum. Dis. 2021, 80, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. RELIEF investigators. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Bazdyrev, E.; Rusina, P.; Panova, M.; Novikov, F.; Grishagin, I.; Nebolsin, V. Lung Fibrosis after COVID-19: Treatment Prospects. Pharmaceuticals 2021, 14, 807. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Vold, A.-M.; Maher, T.; Philpot, E.E.; Ashrafzadeh, A.; Barake, R.; Barsotti, S.; Bruni, C.; Carducci, P.; Carreira, P.E.; Castellví, I.; et al. The identification and management of interstitial lung disease in systemic scleosis: Evidence-based European consensus statements. Lancet Rheumatol. 2020, 2, e71–e83. [Google Scholar] [CrossRef]
- Gono, T.; Sato, S.; Kawaguchi, Y.; Kuwana, M.; Hanaoka, M.; Katsumata, Y.; Takagi, K.; Baba, S.; Okamoto, Y.; Ota, Y.; et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology 2012, 51, 1563–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gono, T.; Kawaguchi, Y.; Satoh, T.; Kuwana, M.; Katsumata, Y.; Takagi, K.; Masuda, I.; Tochimoto, A.; Baba, S.; Okamoto, Y.; et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology 2010, 49, 1713–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seto, N.; Torres-Ruiz, J.J.; Carmona-Rivera, C.; Pinal-Fernandez, I.; Pak, K.; Purmalek, M.M.; Hosono, Y.; Fernandes-Cerqueira, C.; Gowda, P.; Arnett, N.; et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight 2020, 5, e134189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, T.-H.; Tseng, C.-W.; Wang, K.-L.; Fu, P.-K. Combination Therapy with Rituximab, Tofacitinib and Pirfenidone in a Patient with Rapid Progressive Interstitial Lung Disease (RP-ILD) Due to MDA5 Antibody-Associated Dermatomyositis: A Case Report. Medicina 2021, 57, 1358. https://doi.org/10.3390/medicina57121358
Yen T-H, Tseng C-W, Wang K-L, Fu P-K. Combination Therapy with Rituximab, Tofacitinib and Pirfenidone in a Patient with Rapid Progressive Interstitial Lung Disease (RP-ILD) Due to MDA5 Antibody-Associated Dermatomyositis: A Case Report. Medicina. 2021; 57(12):1358. https://doi.org/10.3390/medicina57121358
Chicago/Turabian StyleYen, Tsai-Hung, Chih-Wei Tseng, Kao-Lun Wang, and Pin-Kuei Fu. 2021. "Combination Therapy with Rituximab, Tofacitinib and Pirfenidone in a Patient with Rapid Progressive Interstitial Lung Disease (RP-ILD) Due to MDA5 Antibody-Associated Dermatomyositis: A Case Report" Medicina 57, no. 12: 1358. https://doi.org/10.3390/medicina57121358
APA StyleYen, T.-H., Tseng, C.-W., Wang, K.-L., & Fu, P.-K. (2021). Combination Therapy with Rituximab, Tofacitinib and Pirfenidone in a Patient with Rapid Progressive Interstitial Lung Disease (RP-ILD) Due to MDA5 Antibody-Associated Dermatomyositis: A Case Report. Medicina, 57(12), 1358. https://doi.org/10.3390/medicina57121358







