Clinical Association between Gout and Parkinson’s Disease: A Nationwide Population-Based Cohort Study in Korea
Abstract
:1. Introduction
2. Materials and Methods
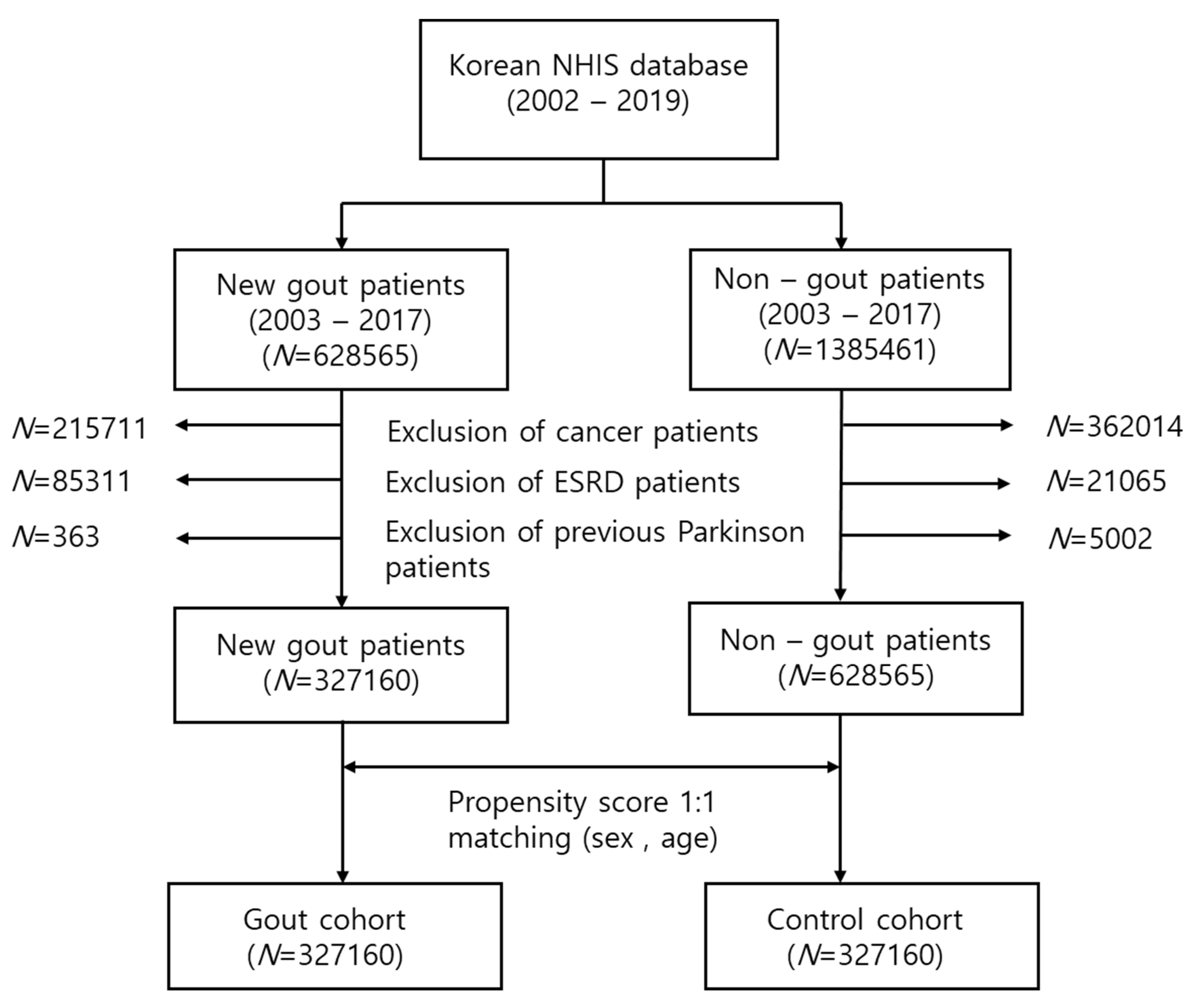
2.1. Database and Study Population
2.2. Study Cohort Selection and Parkinson’s Disease Assessment
2.3. Statistical Analysis
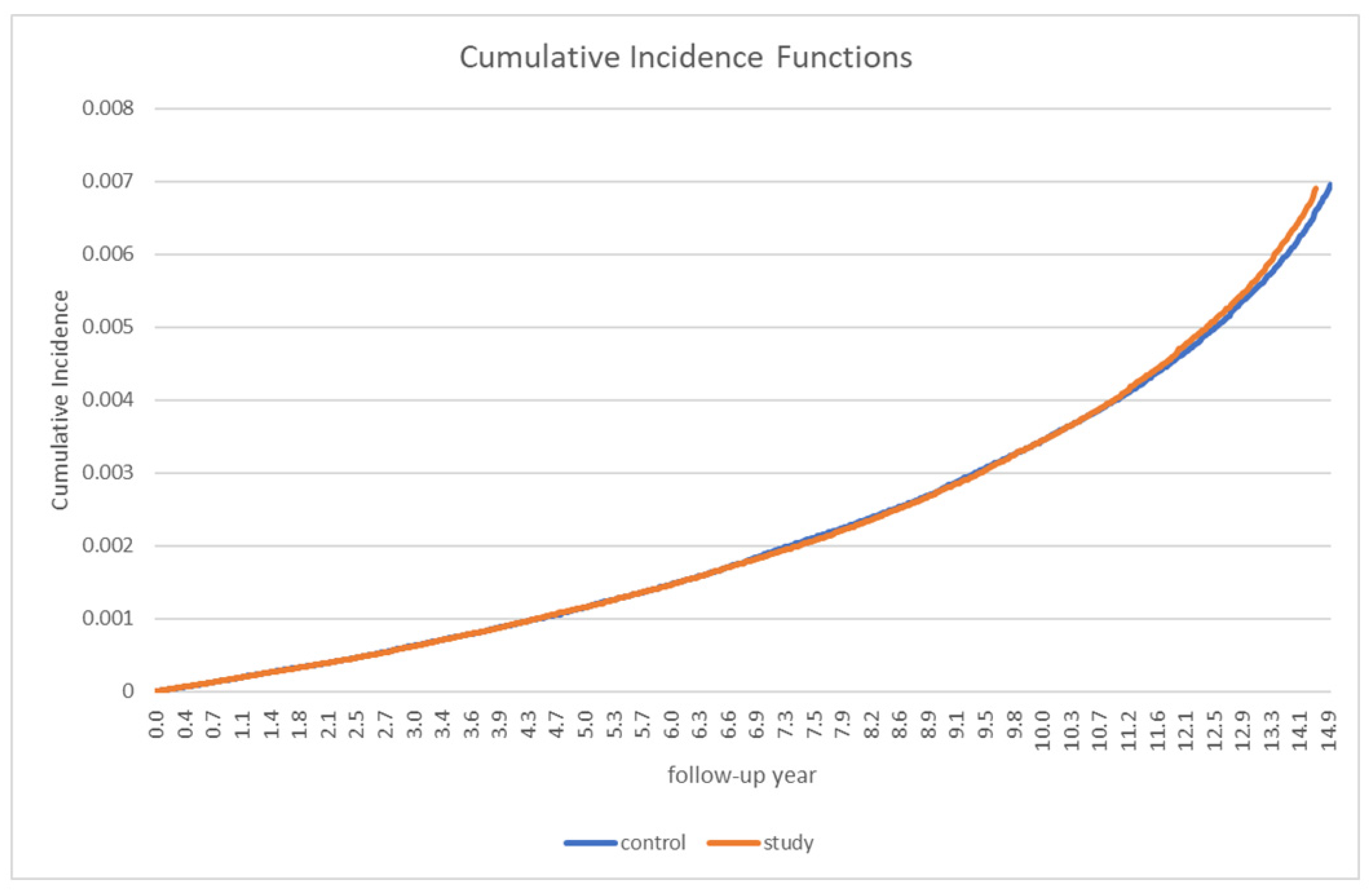
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalia, L.V.; Lang, L.E. Parkinson’s disease in 2015; evolving basic, pathological and clinical concepts in PD. Nat. Rev. Neurol. 2016, 12, 65–66. [Google Scholar] [CrossRef]
- Berardelli, A.; Wenning, G.K.; Antonini, A.; Berg, D.; Bolem, B.R.; Bonfifati, V.; Brooks, D.; Burn, D.J.; Colosimo, C.; Fanciulli, A.; et al. EFNS/MDS-ES/ENS recommendations for the diagnosis of Parkinson’s disease. Eur. J. Neurol. 2013, 20, 16–34. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Roman, G.C. Worldwide occurrence of Parkinson’s disease: An updated review. Neuroepidemiology 1993, 12, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Preingsheim, T.; Jettet, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease; a systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Han, S.L.; Kim, S.; Kim, H.T.; Shin, H.W.; Na, K.S.; Suh, H.S. Prevalence and incidence of Parkinson’s disease and drug-induced parkinsonism in Korea. BMC Public Health 2019, 19, 1328. [Google Scholar] [CrossRef]
- Roddy, E.; Choi, H.K. Epidemiology of gout. Rheum. Dis. Clin. N. Am. 2014, 40, 155–175. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, S.R.; Thomason, H.; Sandler, D.; Leguen, C.; Baxter, M.A.; Thorpe, G.H.G.; Jones, A.F.; Barnett, A.G. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur. J. Clin. Investig. 1997, 27, 484–490. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F.; Hf, J. Low uric acid levels in patients with Parkinson’s disease: Evidence from meta-analysis. BMJ Open 2013, 3, e003620. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.; Drory, V.E. Influence of serum uric acid levels on prognosis and survival in amyotrophic lateral sclerosis: A meta-analysis. J. Neurol. 2014, 261, 1133–1138. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Schiwid, S.R.; Marek, K.; Watts, A.; Lang, A.E.; Oakes, D.; Shoulson, I.; Ascherio, A.; Parkinson Study Group PRECEPT Investigators. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch. Neurol. 2008, 65, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Jenner, P. Oxidative stress as a cause of Parkinson’s disease. Acta Neurol. Scand. Suppl. 1991, 136, 6–15. [Google Scholar] [CrossRef]
- Hwang, O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S36. [Google Scholar] [CrossRef]
- National Health Insurance Service (NHIS). History of the NHIS. Available online: http://www.nhis.or.kr/static/html/wbd/g/a/wbdga0203.html (accessed on 11 April 2016).
- Seong, S.C.; Kim, Y.Y.; Khang, Y.H.; Park, J.H.; Kang, H.J.; Lee, H.Y.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar]
- Singh, J.A.; Cleveland, J.D. Gout and the risk of Parkinson’s disease in older adults: A study of U.S. Medicare data. BMC Neurol. 2019, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Yang, A.C.; Lee, S.C.; You, Z.H.; Tsai, S.J.; Hu, C.K.; Shen, C.C. Risk of Parkinson’s disease following gout: A population-based retrospective cohort study in Taiwan. BMC Neurol. 2020, 20, 338. [Google Scholar] [CrossRef]
- Janssens, H.J.; Arts, P.G.; Schalk, B.W.; Biermans, M.C. Gout and rheumatoid arthritis, both to keep in mind in cardiovascular risk management: A primary care retrospective cohort study. Joint Bone Spine 2017, 84, 59–64. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Kelley, E.E.; Nakagawa, T.; Madero, M.; Feig, D.I.; Borghi, C.; Piani, F.; Cara-Fuentes, G.; Bjornstad, P.; et al. Uric Acid and Hypertension: An Update with Recommendations. Am. J. Hypertens. 2020, 33, 583–594. [Google Scholar] [CrossRef]
- Kutzing, M.K.; Firestein, B.L. Altered uric acid levels and disease states. J. Pharmacol. Exp. Ther. 2008, 324, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piani, F.; Cicero, A.F.G.; Borghi, C. Uric Acid and Hypertension: Prognostic Role and Guide for Treatment. J. Clin. Med. 2021, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Nishikawa, S.; Takahashi, K. Epidemiology of Parkinson’s disease in a Japanese city. Arch. Neurol. 1983, 40, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Li, C.Y.; Lee, P.C.; Sun, Y. Variations in incidence and prevalence of Parkinson’s disease in Taiwan: A population-based nationwide study. Parkinson’s Dis. 2016, 2016, 8756359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shi, Y.; Wu, Z.; He, Y.; Zhang, B. Parkinson’s disease in China. Chin. Med. J. 1991, 104, 960–964. [Google Scholar]
- Benito-Leon, J.; Bermejo-Pareja, F.; Morales-Gonzalez, J.; Porta-Etessam, J.; Trincado, R.; Vega, S.; Louis, E.D. Incidence of Parkinson disease and parkinsonism in three elderly populations of Central Spain. Neurology 2004, 62, 734–741. [Google Scholar] [CrossRef]
- Nerius, M.; Fink, A.; Doblhammer, G. Parkinson’s disease in Germany: Prevalence and incidence based on health claims data. Acta Neurol. Scand. 2017, 136, 386–392. [Google Scholar] [CrossRef]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinson’s Dis. 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Bauso, D.; Tartari, J.; Stefani, C.; Rojas, J.; Giunta, D.; Cristiano, E. Incidence and prevalence of Parkinson’s disease in Buenos Aires City. Argent. Eur. J. Neurol. 2012, 19, 1108–1113. [Google Scholar] [CrossRef]
- Hu, G.; Antikainen, R.; Jousilahti, P.; Kivipelto, M.; Tuomilehto, J. Total cholesterol and the risk of Parkinson disease. Neurology 2008, 70, 1972–1979. [Google Scholar] [CrossRef]
- Guo, X.; Song, W.; Chen, K.; Chen, X.; Zheng, Z.; Cao, B.; Huang, R.; Zhao, B.; Wu, Y.; Shang, H.-F. The serum lipid profile of Parkinson’s disease patients: A study from China. Int. J. Neurosci. 2015, 125, 838–844. [Google Scholar] [CrossRef]
- Rozani, V.; Gurevich, T.; Giladi, N.; El-Ad, B.; Tsamir, J.; Hemo, B.; Peretz, C. Higher serum cholesterol and decreased Parkinson’s disease risk: A statin-free cohort study. Mov. Disord. 2018, 33, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhan, Y.; Hammar, N.; Shen, X.; Wirdefeldt, K.; Walldius, G.; Mariosa, D. Lipids, apolipoproteins, and the risk of Parkinson disease. Circ. Res. 2019, 125, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Auinger, P.; Eberly, S.; Oakes, D.; Schwarzschild, M.; Ascherio, A.; Mailman, R.; Chen, H.; Parkinson Study Group DATATOP Investigators. Serum Cholesterol and the Progression of Parkinson’s Disease: Results from DATATOP. PLoS ONE 2011, 6, e22854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barres, B.A.; Smith, S.J. Cholesterol—Making or breaking the synapse. Science 2001, 294, 12961297. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.; Hannah, M.J.; Fahrenholz, F.; Huttner, W.B. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat. Cell Biol. 2000, 2, 4249. [Google Scholar] [CrossRef]
- Stoffel-Wagner, B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001, 145, 669679. [Google Scholar] [CrossRef] [Green Version]
- Rouge-Pont, F.; Mayo, W.; Marinelli, M.; Gingras, M.; Le Moal, M.; Piazza, P.V. The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur. J. Neurosci. 2002, 16, 169173. [Google Scholar] [CrossRef]
- Barrot, M.; Vallee, M.; Gingras, M.A.; Le Moal, M.; Mayo, W.; Piazza, P.V. The neurosteroid pregnenolone sulphate increases dopamine release and the dopaminergic response to morphine in the rat nucleus accumbens. Eur. J. Neurosci. 1999, 11, 37573760. [Google Scholar] [CrossRef]
- Shalaby, S.Y.; Louis, E.D. Statin use and its association with essential tremor and Parkinson’s disease. Neuroepidemiology 2016, 47, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Seng, Z.; Jia, X.; Kang, M. Statin use and risk of Parkinson’s disease: A meta-analysis. Behav. Brain Res. 2016, 309, 29–34. [Google Scholar] [CrossRef]
- Belvisi, D.; Pellicciari, R.; Fabbrini, G.; Tinazzi, M.; Berardelli, A.; Defazio, G. Modifiable risk and protective factors in disease development, progression and clinical subtypes of Parkinson’s disease: What do prospective studies suggest. Neurobiol. Dis. 2020, 134, 104671. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Sevanian, A.; Muakkassah-Kelly, S.F.; Hochstein, P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem. J. 1986, 235, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Waring, W.S. Uric acid: An important antioxidant in acute ischemic stroke. QJM 2002, 95, 691–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Jin, X.; Guo, D.; Tong, G.; Zhu, X. Iron chelation nanoparticles with delayed saturation as an effective therapy for Parkinson Disease. Biomacromolecules 2017, 18, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Dusek, P.; Schneider, S.A.; Aaseth, J. Iron chelation in the treatment of neurodegenerative diseases. J. Trace Elem. Med. Biol. 2016, 38, 81–92. [Google Scholar] [CrossRef]
| Variables | Gout Patients | Controls | p-Value |
|---|---|---|---|
| (N = 327,160) | (N = 327,160) | ||
| Sex Male Female | 304,162 22,998 | 304,162 22,998 | |
| Age (years) | |||
| <30 | 30,581 | 30,581 | |
| 30–39 | 75,265 | 75,265 | |
| 40–49 | 85,730 | 85,730 | |
| 50–59 | 70,912 | 70,912 | |
| 60–69 | 39,063 | 39,063 | |
| 70–79 | 19,638 | 19,638 | |
| ≥80 | 5.971 | 5.971 | |
| Underlying diseases | |||
| Hypertension | 211,097 (64.52%) | 140,890 (43.06%) | <0.001 |
| Diabetes | 186,198 (56.91%) | 130,351(39.84%) | <0.001 |
| Dyslipidemia | 284,794 (87.05%) | 203,823 (62.3%) | <0.001 |
| Ischemic heart disease | 102,591(31.36%) | 73,162 (22.36%) | <0.001 |
| Stroke | 34,920 (10.67%) | 27,777 (8.49%) | <0.001 |
| Variables | Gout (N = 327,160) | Controls (N = 327,160) | IRR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| N, % | PY | IR | N, % | PY | IR | ||
| Overall | 912 | 6544.07 | 0.35 | 929 | 6676.18 | 0.36 | 0.98 (0.89–1.07) |
| Sex | |||||||
| Male | 718 | 5437.98 | 0.29 | 748 | 5541.36 | 0.31 | 0.98 (0.88–1.08) |
| Female | 194 | 1106.09 | 1.32 | 181 | 1134.82 | 1.19 | 1.09 (0.9–1.35) |
| Age, years | |||||||
| 20–29 | 2 | 8.18 | 0.01 | 3 | 11.45 | 0.01 | 0.67 (0.11–4.0) |
| 30–39 | 14 | 87.16 | 0.02 | 17 | 139.21 | 0.03 | 0.82 (0.41–1.67) |
| 40–49 | 60 | 441.84 | 0.08 | 66 | 524.01 | 0.09 | 0.91 (0.64–1.28) |
| 50–59 | 164 | 1363.84 | 0.28 | 170 | 1334.36 | 0.3 | 0.95 (0.77–1.18) |
| 60–69 | 325 | 2550.98 | 1.10 | 312 | 2475.13 | 1.07 | 1.03 (0.88–1.2) |
| 70–79 | 277 | 1779.07 | 0.56 | 299 | 1952.53 | 2.41 | 0.91 (0.78–1.08) |
| ≥80 | 70 | 313.01 | 2.53 | 62 | 239.48 | 2.40 | 1.05 (0.75–1.48) |
| Unadjusted HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Gout | 0.98 (0.89–1.07) | 0.602 | 1.00 (0.91–1.10) | 0.935 |
| Female sex | 4.731 (4.22–5.30) | <0.001 | 1.21 (1.07–1.37) | 0.002 |
| Age | ||||
| 30–39 | 2.29 (0.89–5.9) | 0.085 | 2.33 (0.91–6.00) | 0.079 |
| 40–49 | 7.58 (3.10–18.54) | <0.001 | 7.7 (3.15–18.83) | <0.001 |
| 50–59 | 27.63 (43.35–251.96) | <0.001 | 26.95 (11.12–65.34) | <0.001 |
| 60–69 | 104.51 (43.35–251.96) | <0.001 | 92.08 (38.03–222.93) | <0.001 |
| 70–79 | 256.07 (106.17–617.61) | <0.001 | 197.5 (81.39–479.21) | <0.001 |
| ≥80 | 343.41 (140.52–839.22) | <0.001 | 235.26 (95.53–579.38) | <0.001 |
| Hypertension | 3.59 (3.17–4.03) | <0.001 | 1.16 (1.01–1.34) | 0.040 |
| Diabetes | 2.02 (1.83–2.22) | <0.001 | 0.93 (0.83–1.04) | 0.216 |
| Dyslipidemia | 1.25 (1.11–1.40) | <0.001 | 0.6 (0.52–0.68) | <0.001 |
| Ischemic heart disease | 2.37 (2.17–2.60) | <0.001 | 0.99 (0.90–1.1) | 0.998 |
| Stroke | 5.71 (5.2–6.27) | <0.001 | 1.95 (1.76–2.16) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Choi, I.A.; Kim, A.; Kang, G. Clinical Association between Gout and Parkinson’s Disease: A Nationwide Population-Based Cohort Study in Korea. Medicina 2021, 57, 1292. https://doi.org/10.3390/medicina57121292
Kim JH, Choi IA, Kim A, Kang G. Clinical Association between Gout and Parkinson’s Disease: A Nationwide Population-Based Cohort Study in Korea. Medicina. 2021; 57(12):1292. https://doi.org/10.3390/medicina57121292
Chicago/Turabian StyleKim, Ji Hyoun, In Ah Choi, Aryun Kim, and Gilwon Kang. 2021. "Clinical Association between Gout and Parkinson’s Disease: A Nationwide Population-Based Cohort Study in Korea" Medicina 57, no. 12: 1292. https://doi.org/10.3390/medicina57121292
APA StyleKim, J. H., Choi, I. A., Kim, A., & Kang, G. (2021). Clinical Association between Gout and Parkinson’s Disease: A Nationwide Population-Based Cohort Study in Korea. Medicina, 57(12), 1292. https://doi.org/10.3390/medicina57121292






