Exertional Desaturation Has Higher Mortality Than Non-Desaturation in COPD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Ethical Approval and Patient Informed Consent
2.4. Statistics
3. Results
3.1. The Characteristics of the Participants
3.2. Risk Factors with ED
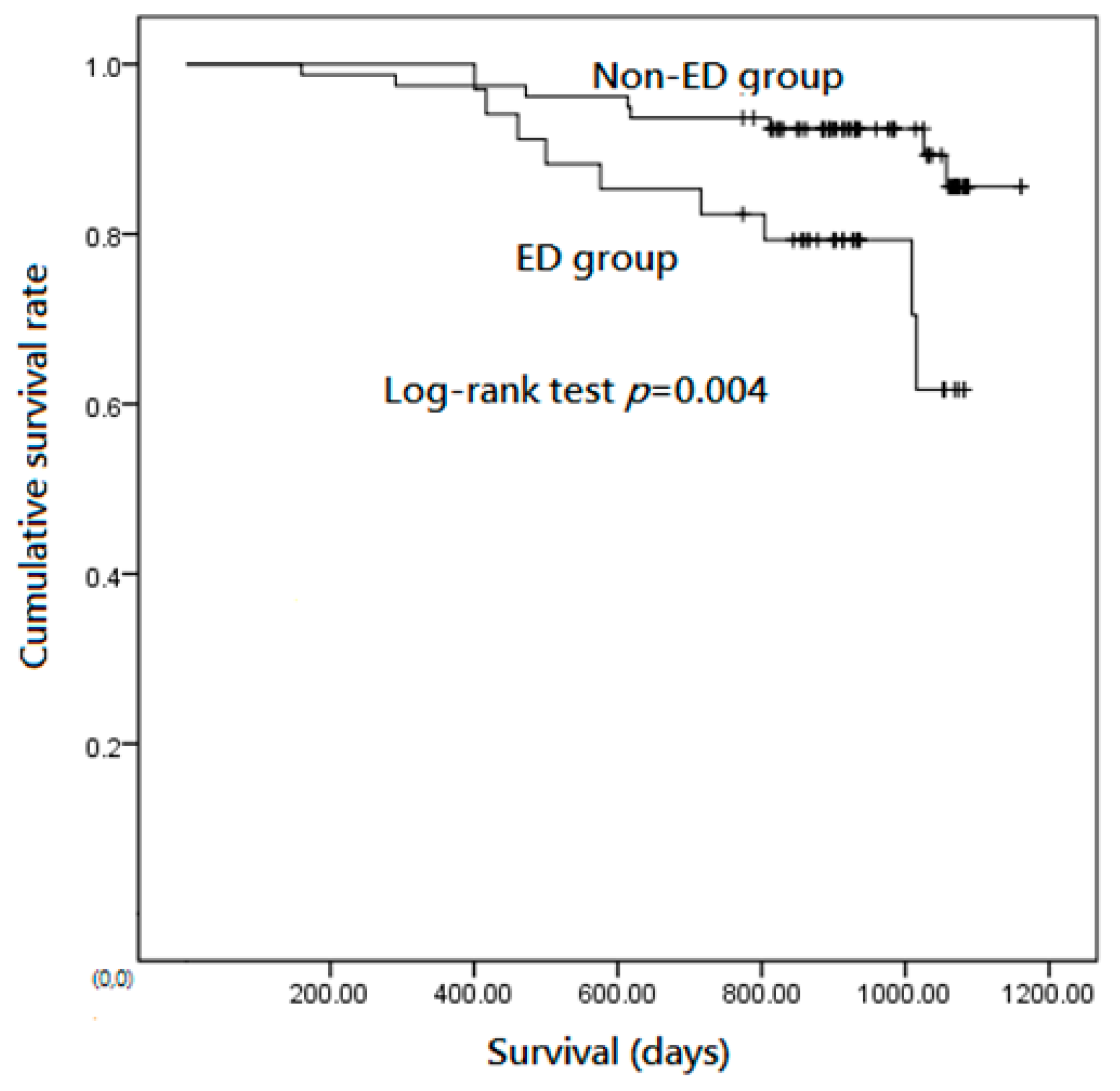
3.3. ED Is a Predictor of Mortality in Patients with Stable COPD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ED | exertional desaturation |
| COPD | chronic obstructive pulmonary disease |
| HRs | hazard ratios |
| DLCO | diffusing capacity of the lungs for carbon monoxide |
| 6MWT | 6 minute walking test |
| hsCRP | serum high-sensitivity C-reactive protein |
| V | ventilation |
| Q | perfusion ratio |
| MMRC | Modified Medical Research Council dyspnea scale |
| BMI | body mass index |
| BODE | body mass index, airflow obstruction, dyspnea, and exercise capacity |
| FVC | forced vital capacity |
| FEV1 | forced expiratory volume in 1 s |
| RR | respiratory rate; |
| SpO2 | oxyhemoglobin saturation by pulse oximetry. |
References
- Stolz, D.; Boersma, W.; Blasi, F.; Louis, R.; Milenkovic, B.; Kostikas, K.; Aerts, J.G.; Rohde, G.; Lacoma, A.; Rakic, J.; et al. Exertional hypoxemia in stable COPD is common and predicted by circulating proadrenomedullin. Chest 2014, 146, 328–338. [Google Scholar] [CrossRef]
- Panos, R.J.; Eschenbacher, W. Exertional desaturation in patients with chronic obstructive pulmonary disease. COPD J. Chronic Obstr. Pulm. Dis. 2009, 6, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 199. [Google Scholar]
- Ussetti, P.; Rodriguez-Roisin, R. Gas exchange during exercise in mild chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1991, 144, 520–525. [Google Scholar]
- Dantzker, D.R.; D’Alonzo, G.E. The effect of exercise on pulmonary gas exchange in patients with severe chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1986, 134, 1135–1139. [Google Scholar]
- O’Donnell, D.E.; D’Arsigny, C.; Fitzpatrick, M.; Webb, K.A. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: The role of lung hyperinflation. Am. J. Respir. Crit. Care Med. 2002, 166, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Knower, M.T.; Dunagan, D.P.; Adair, N.E.; Chin, R., Jr. Baseline oxygen saturation predicts exercise desaturation below prescription threshold in patients with chronic obstructive pulmonary disease. Arch. Intern. Med. 2001, 161, 732–736. [Google Scholar] [CrossRef] [Green Version]
- Cutaia, M.; Brehm, R.; Cohen, M. The relationship of the BODE index to oxygen saturation during daily activities in patients with chronic obstructive pulmonary disease. Lung 2011, 189, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.A.; Tsuang, W.; Lach, L.; Eschenbacher, W.; Panos, R.J. Dynamic hyperinflation correlates with exertional oxygen desaturation in patients with chronic obstructive pulmonary disease. Lung 2013, 191, 177–182. [Google Scholar] [CrossRef]
- Minh, V.-D.; Lee, H.M.; Dolan, G.F.; Light, R.W.; Bell, J.; Vasquez, P. Hypoxemia during exercise in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1979, 120, 787–794. [Google Scholar] [PubMed]
- Nowiński, A.; Kamiński, D.; Kram, M.; Korzybski, D.; Stokłosa, A.; Górecka, D. Impact of mild anaemia on dyspnoea during exertion and exercise tolerance in patients with acute exacerbation of chronic obstructive pulmonary disease. Adv. Respir. Med. 2013, 81, 200–206. [Google Scholar]
- Moreira, M.Â.F.; Medeiros, G.A.D.; Boeno, F.P.; Sanches, P.R.S.; Silva, D.P.D.; Müller, A.F. Oxygen desaturation during the six-minute walk test in COPD patients. J. Bras. Pneumol. 2014, 40, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Seo, J.B.; Lee, S.Y.; Lee, J.S.; Huh, J.W.; Lee, J.H.; Ra, S.W.; Lee, J.-H.; Kim, E.-K.; Kim, T.-H.; et al. Exertional desaturation as a predictor of rapid lung function decline in COPD. Respiration 2013, 86, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Couillard, A.; Muir, J.F.; Veale, D. COPD recent findings: Impact on clinical practice. COPD J. Chronic Obstr. Pulm. Dis. 2010, 7, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Tojo, N.; Ichioka, M.; Chida, M.; Miyazato, I.; Yoshizawa, Y.; Miyasaka, N. Pulmonary exercise testing predicts prognosis in patients with chronic obstructive pulmonary disease. Intern. Med. 2005, 44, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova, C.; Cote, C.; Marin, J.M.; Pinto-Plata, V.; de Torres, J.P.; Aguirre-Jaíme, A.; Vassaux, C.; Celli, B.R. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008, 134, 746–752. [Google Scholar] [CrossRef]
- Stoller, J.K.; Panos, R.J.; Krachman, S.; Doherty, D.E.; Make, B.; Group L-tOTTR. Oxygen therapy for patients with COPD: Current evidence and the long-term oxygen treatment trial. Chest 2010, 138, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Group L-TOTTR. A randomized trial of long-term oxygen for COPD with moderate desaturation. N. Engl. J. Med. 2016, 375, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Ameer, F.; Carson, K.V.; Usmani, Z.A.; Smith, B.J. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst. Rev. 2014, 2014, CD000238. [Google Scholar] [CrossRef] [PubMed]
- GROUP* NOTT. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann. Intern. Med. 1980, 93, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Stuart, H.; Harris, S.; Tjh, C.; Dornhorst, A.C.; Cotes, J.E.; Flenley, D.C.; Howard, P.; Oldham, P.D. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981, 1, 681–685. [Google Scholar]
- The Global Initiative for Chronic Obstructive Lung Disease: GOLD. Available online: http://www.goldcopd.com (accessed on 10 September 2021).
- Celli, B.; Cote, C.; Marín, J.; Casanova, C.; Montes de Oca Mendez, R. The body mass index, airflow obstruction, dyspnea, exercise performance (BODE) index as a predictor of mortality in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulain, M.; Durand, F.; Palomba, B.; Ceugniet, F.; Desplan, J.; Varray, A. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003, 123, 1401–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.M.; Cho, J.G.; Sandoz, J.S.; Wheatley, J.R. Oxygen desaturation and adverse events during 6-min walk testing in patients with COPD. Respirology 2015, 20, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto-Plata, V.; Cote, C.; Cabral, H.; Taylor, J.; Celli, B. The 6-min walk distance: Change over time and value as a predictor of survival in severe COPD. Eur. Respir. J. 2004, 23, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Gould, G.; Redpath, A.; Ryan, M.; Warren, P.; Best, J.; Flenley, D.; MacNee, W. Lung CT density correlates with measurements of airflow limitation and the diffusing capacity. Eur. Respir. J. 1991, 4, 141–146. [Google Scholar]
- Gould, G.A.; Redpath, A.T.; Ryan, M.; Warren, P.M.; Best, J.J.; Flenley, D.C.; MacNee, W. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am. J. Respir. Crit. Care Med. 1996, 154, 187–192. [Google Scholar]
- Sue, D.Y.; Oren, A.; Hansen, J.E.; Wasserman, K. Diffusing capacity for carbon monoxide as a predictor of gas exchange during exercise. N. Engl. J. Med. 1987, 316, 1301–1306. [Google Scholar] [CrossRef]
- Hadeli, K.O.; Siegel, E.M.; Sherrill, D.L.; Beck, K.C.; Enright, P.L. Predictors of oxygen desaturation during submaximal exercise in 8,000 patients. Chest 2001, 120, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Soguel Schenkel, N.; Burdet, L.; de Muralt, B.; Fitting, J.W. Oxygen saturation during daily activities in chronic obstructive pulmonary disease. Eur. Respir. J. 1996, 9, 2584–2589. [Google Scholar] [CrossRef]
- Waatevik, M.; Johannessen, A.; Real, F.G.; Aanerud, M. Oxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPD. Eur. Respir. J. 2016, 48, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.; Lee, A.; McDonald, C.; Hill, C.J.; Holland, A.E. Should oxyhaemoglobin saturation be monitored continuously during the 6-minute walk test? Chronic Respir. Dis. 2011, 8, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L. Oxygen desaturation during a 6-min walk identifies a COPD phenotype with an increased risk of morbidity and mortality. Eur. Respir. J. 2016, 48, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.-F.; Wang, C.-C.; Chin, C.-H.; Chen, Y.-C.; Lin, M.-C. High value of combined serum C-reactive protein and BODE score for mortality prediction in patients with stable COPD. Arch. Bronconeumol. 2011, 47, 427–432. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Mean ± SD |
|---|---|
| Age (y) | 69.5 ± 10.3 |
| Male (%) | 110/113 (97.3) |
| Smoking history (pack-y) | 58.0 ± 31.8 |
| Current smoking status (%) | 35/113 (31.0) |
| Body mass index (BMI) | 23.5 ± 3.7 |
| FVC (% of predicted value) | 71.7 ± 19.3 |
| FEV1/FVC (%) | 53.9 ± 11.5 |
| FEV1 (% of predicted value) | 53.1 ± 21.5 |
| DLCO (%) | 72.3 ± 24.2 |
| Old GOLD stage | |
| I/II/III/IV | 17//35/50/11 |
| 2012 GOLD | |
| Group A/B/C/D | 31/22//10/50 |
| mMRC dyspnea scale ‡ | |
| Scale 0/1/2/3/4 | 17/25/29/30/31 |
| 6MWD (m) | 402.4 ± 111.1 |
| ED Group (N = 34) | Non-ED Group (N = 79) | p (95% CI) | p * (95% CI) | |
|---|---|---|---|---|
| Age (Mean ± SD) | 68.3 ± 9.7 | 70.1 ± 10.6 | 0.41 | |
| Male (%) | 97 ± 17 | 97 ± 15 | 0.90 | |
| Smoking history(n)(%) | 34(100) | 79(100) | - | |
| Pack-yrs (Mean ± SD) | 58.5 ± 33.7 | 57.7 ± 31.1 | 0.52 | |
| Current smoking (n)(%) | 10(29.5) | 26(33) | 0.91 | |
| Body mass index (BMI) | 23.2 ± 3.9 | 23.7 ± 3.6 | 0.79 | |
| FVC (% of predicted value) | 65.7 ± 19.3 | 74.3 ± 18.9 | 0.03 −16.0–0.3 | 0.80 −0.001–0.01 |
| FEV1/FVC (%) | 48.7 ± 11.1 | 56.1 ± 11.1 | 0.02 −16.2–−0.2 | 0.70 −0.19–0.13 |
| FEV1 (% of predicted value) | 43.7 ± 17.8 | 57.2 ± 21.7 | 0.02 −23.4–−6.1 | 0.9 −0.14–0.02 |
| DLCO (%) | 58.0 ± 17.0 | 78.4 ± 24.4 | <0.001 −31.6–−12.6 | 0.5 −0.004–0.002 |
| 6MWD (m) | 391.8 ± 109.9 | 411.6 ± 104.6 | 0.37 | |
| MIP | 75.3 ± 22.9 | 87.8 ± 31.8 | 0.04 −26.6–−1.9 | 0.76 −0.02–0.002 |
| MEP | 97.7 ± 31.6 | 99.2 ± 34.8 | 0.82 | |
| SpO2 (initial) * | 96.1 ± 1.9 | 96.6 ± 1.3 | 0.15 | |
| SpO2 (end) # | 95.1 ± 4.2 | 96.2 ± 1.5 | 0.06 −2.2–0.03 | 0.5 −0.02–0.03 |
| Minimal SpO2 | 84.2 ±5.4 | 93.0 ±1.7 | <0.001 −10.6–−7.8 | <0.001 −0.08–−0.05 |
| HR (initial) * | 82.9 ± 16.0 | 80.2 ± 15.1 | 0.4 | |
| HR (end) # | 100.1 ± 17.2 | 94.9 ± 16.0 | 0.1 −1.3–12.3 | |
| Maximal HR | 136.8 ±21.5 | 120.9 ±19.1 | <0.001 9.4–26.0 | 0.04 0.00–0.07 |
| RR (initial) * | 14.9 ± 3.5 | 14.7 ± 3.2 | 0.78 | |
| RR (end) # | 18.7 ± 5.1 | 17.0 ± 4.1 | 0.06 0.4–3.8 | 0.65 −0.02–0.01 |
| Borg scale (initial) * | 2.0 ± 1.0 | 2.1 ±1.1 | 0.78 | |
| Borg scale (end) # | 2.9 ±1.2 | 2.8 ± 1.4 | 0.60 | |
| ΔBorg scale | 0.94 ± 0.2 | 0.73 ± 0.1 | 0.32 | |
| Hs-CRP | 3.8 ± 0.7 | 3.1 ± 0.4 | 0.37 | |
| fibrinogen | 319.3 ± 84.5 | 303.6 ± 76.0 | 0.34 | |
| 2012 GOLDGroup A/B/C/D | 4/5/4/21 | 27/17/6/29 | 0.003 0.4–1.4 | 0.78 −0.09–0.11 |
| FEV1 severity | 2/7/16/9 | 15/28/34/2 | <0.001 0.4–1.0 | 0.83 −2.35–0.19 |
| MMRC | 1/7/10/11/5 | 15/18/19/19/8 | 0.04 0.2–1.2 | 0.92 −0.07–0.07 |
| Acute exacerbation | 0.32 ± 0.08 | 0.23 ± 0.05 | 0.29 |
| p ^ | Exp(B) ^ | 95% CI ^ | p * | Exp(B) * | 95% CI * | |
|---|---|---|---|---|---|---|
| Age | 0.023 | 1.06 | 1.01–1.12 | 0.028 | 1.08 | 1.01–1.17 |
| Gender | 0.38 | 0.05 | 0.00–60694 | 0.99 | 0 | 0 |
| BMI | 0.13 | 0.89 | 0.78–1.03 | 0.79 | 0.98 | 0.84–1.15 |
| 6MWD | 0.01 | 0.995 | 0.991–0.995 | 0.82 | 1.001 | 0.99–1.01 |
| FEV1(%) | 0.55 | 0.99 | 0.99–1.02 | 0.88 | 1.004 | 0.95–1.06 |
| MMRC scale | 0.008 | 1.75 | 1.16–2.66 | 0.027 | 2.01 | 1.08–3.71 |
| GOLD stage | 0.32 | 1.22 | 0.82–1.84 | 0.82 | 1.001 | 0.995–1.01 |
| AE | 0.81 | 0.93 | 0.31–2.84 | 0.99 | 1.01 | 0.24–4.28 |
| ED | 0.007 | 3.68 | 0.11–0.70 | 0.012 | 4.12 | 1.37–12.39 |
| hS-CRP < 3 mg/L | 0.013 | 0.22 | 0.60–0.82 | 0.017 | 0.22 | 0.06–0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-F.; Chin, C.-H.; Tseng, C.-W.; Chen, Y.-C.; Kuo, H.-C. Exertional Desaturation Has Higher Mortality Than Non-Desaturation in COPD. Medicina 2021, 57, 1110. https://doi.org/10.3390/medicina57101110
Liu S-F, Chin C-H, Tseng C-W, Chen Y-C, Kuo H-C. Exertional Desaturation Has Higher Mortality Than Non-Desaturation in COPD. Medicina. 2021; 57(10):1110. https://doi.org/10.3390/medicina57101110
Chicago/Turabian StyleLiu, Shih-Feng, Chien-Hung Chin, Ching-Wang Tseng, Yung-Che Chen, and Ho-Chang Kuo. 2021. "Exertional Desaturation Has Higher Mortality Than Non-Desaturation in COPD" Medicina 57, no. 10: 1110. https://doi.org/10.3390/medicina57101110
APA StyleLiu, S.-F., Chin, C.-H., Tseng, C.-W., Chen, Y.-C., & Kuo, H.-C. (2021). Exertional Desaturation Has Higher Mortality Than Non-Desaturation in COPD. Medicina, 57(10), 1110. https://doi.org/10.3390/medicina57101110







