Lemierre’s Syndrome: Case Presentation of a Rare and Possibly Life-Threatening Condition
Abstract
:1. Introduction
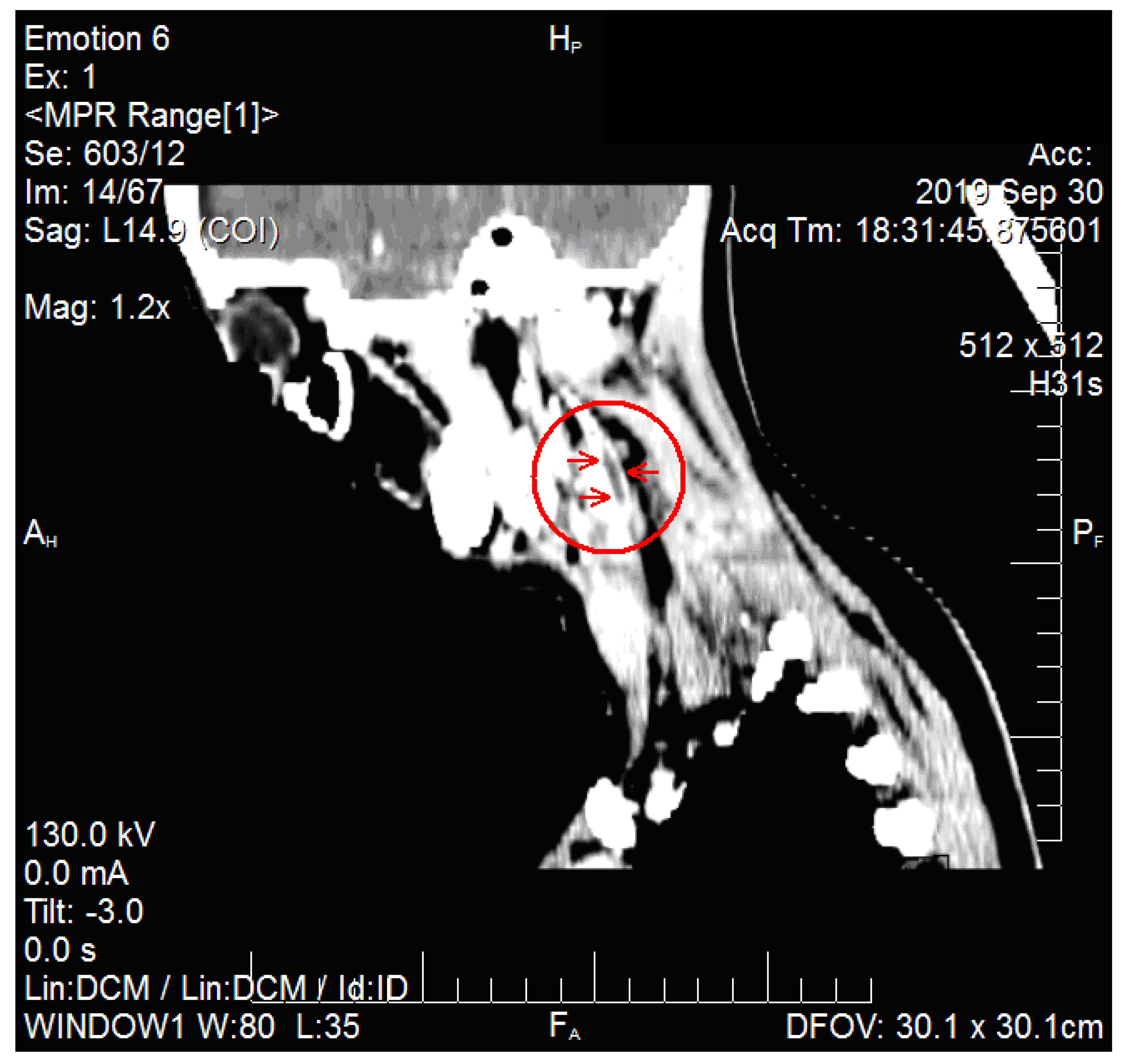
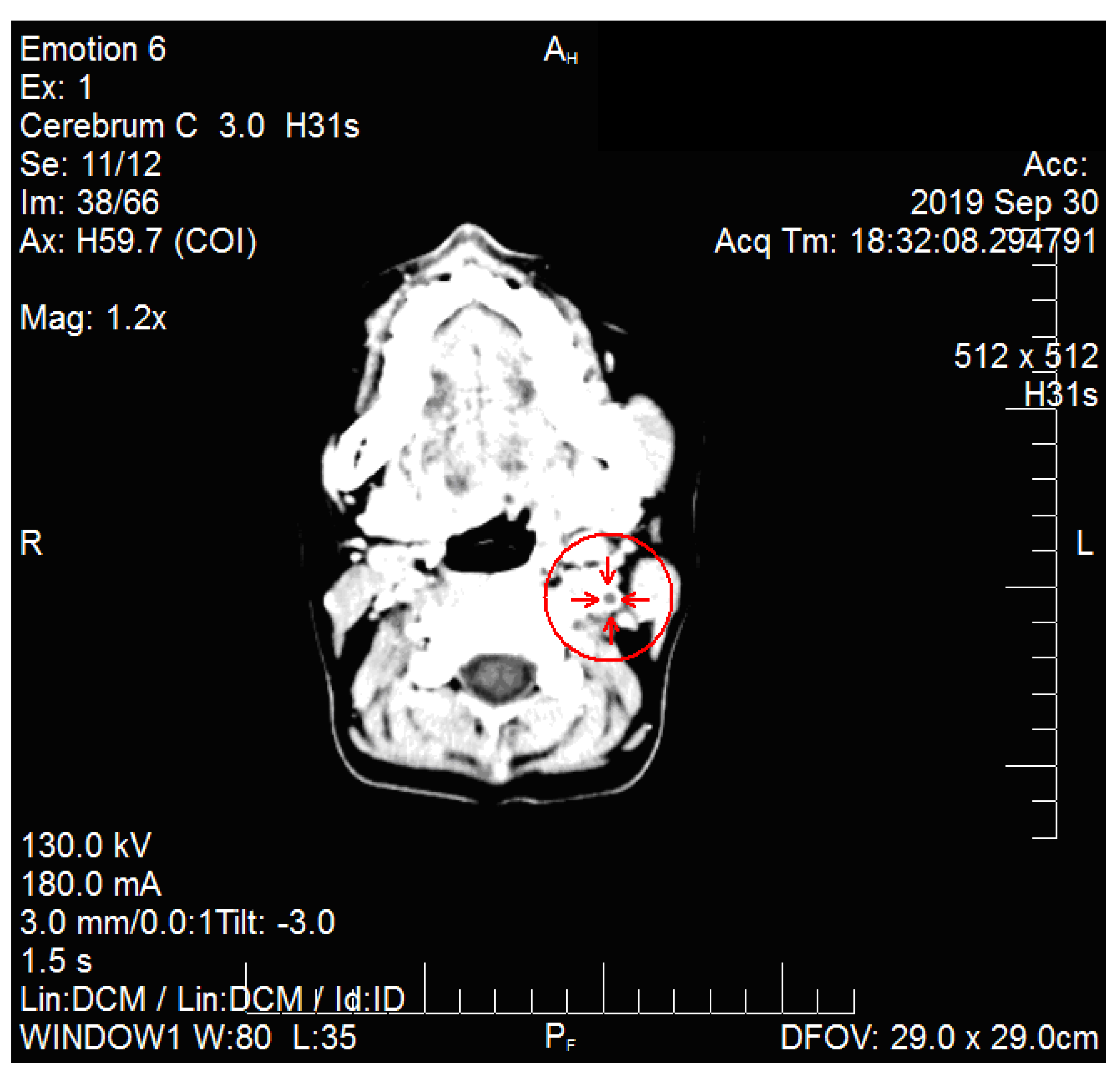
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lemierre, A. On certain septicaemias due to anaerobic organisms. Lancet 1936, 227, 701–703. [Google Scholar] [CrossRef]
- Siddique, M.K.; Chang, G.; Lagmay, V.; Shih, M. Lemierre’s syndrome caused by Streptococcus pyogenes in an elderly woman. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 31–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surapaneni, B.K.; Omar, H.; Iguina, M.M.; Suarez, M. Fusobacterium necrophorum Septicemia Leading to Lemierre’s Syndrome in an Immunocompetent Individual: A Case Report. Cureus 2020, 12, e7443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, A.; Dimitriou, D.; Dimitriou, P.; Katsios, A.; Petrikkos, G. Complicated Lemierre Syndrome Caused by Streptococcus gordonii and Possible Rickettsial Co-Infection in a Patient with Thrombophilia Predisposition. Eur. J. Case Rep. Intern. Med. 2017, 2, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, L.; Corsi, G.; Sebastian, T.; Barco, S. Lemierre syndrome: Current evidence and rationale of the Bacteria-Associated Thrombosis, Thrombophlebitis and Lemierre syndrome (BATTLE) registry. Thromb. Res. 2020, 196, 494–499. [Google Scholar] [CrossRef] [PubMed]
- al Duwaiki, S.M.; al Barwani, A.S.; Taif, S. Lemierre’s Syndrome. Oman Med. J. 2018, 33, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Herwaldt, L. Lemierre’s syndrome: A re-emerging infection. IDCases 2020, 19, e00668. [Google Scholar] [CrossRef] [PubMed]
- Laurencet, M.-E.; Rosset-Zufferey, S.; Schrenzel, J. Atypical presentation of Lemierre’s syndrome: Case report and literature review. BMC Infect. Dis. 2019, 19, 868. [Google Scholar] [CrossRef] [PubMed]
- Noy, D.; Rachmiel, A.; Levy-Faber, D.; Emodi, O. Lemierre’s syndrome from odontogenic infection: Review of the literature and case description. Ann. Maxillofac. Surg. 2015, 5, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campo, F.; Fusconi, M.; Ciotti, M.; Diso, D.; Greco, A.; Cattaneo, C.G.; de Vincentiis, M. Antibiotic and Anticoagulation Therapy in Lemierre’s Syndrome: Case Report and Review. J. Chemother. 2019, 31, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.; Ramsay, G.; et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Siloşi, C.A.; Siloşi, I.; Pădureanu, V.; Bogdan, M.; Mogoantă, S.S.; Ciurea, M.E.; Cojocaru, M.; Boldeanu, L.; Avrămescu, C.S.; Bolde-anu, M.V.; et al. Sepsis and identification of reliable biomarkers for postoperative period prognosis. Rom. J. Morphol. Embryol. 2018, 59, 77–91. [Google Scholar] [PubMed]
- Dragoescu, A.N.; Padureanu, V.; Stanculescu, A.D.; Chiutu, L.C.; Florescu, D.N.; Gheonea, I.A.; Padureanu, R.; Stepan, A.; Streba, C.T.; Drocas, A.I.; et al. Presepsin as a Potential Prognostic Marker for Sepsis According to Actual Practice Guidelines. J. Pers. Med. 2021, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- 14 Valerio, L.; Zane, F.; Sacco, C.; Granziera, S.; Nicoletti, T.; Russo, M.; Corsi, G.; Holm, K.; Hotz, M.; Righini, C.; et al. Patients with Lemierre syndrome have a high risk of new thromboembolic complication, clinical sequelae and death: An analysis of 712 cases. J. Intern. Med. 2020, 289, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Cupit-Link, M.C.; Rao, A.N.; Warad, D.M.; Rodriguez, V. Lemierre Syndrome: A Retrospective Study of the Role of Anticoagulation and Thrombosis Outcomes. Acta Haematol. 2017, 137, 59–65. [Google Scholar] [CrossRef] [PubMed]
| Laboratory Test | Value | Reference Range |
|---|---|---|
| Hemoglobin (g/L) | 11.3 | 11–15 |
| White blood cells count (×103/mm3) | 21.4 | 4–9 |
| Neutrophils (%) | 81.2 | 50–68 |
| Thrombocytes (×103/mm3) | 498 | 150–300 |
| Erythrocyte sedimentation rate (mm/1 h) | 120 | 12–20 |
| Urea (mg/dL) | 23.5 | 20–40 |
| Glucose (mg/dL) | 91.7 | 80–115 |
| TGP (u/L) | 14.9 | 10–35 |
| Urinalysis | normal | – |
| Fibrinogen (mg/dL) | 550 | 200–400 |
| C-reactive protein (mg/L) | 192 | <10 |
| Procalcitonin (ng/mL, semiquantitative) | 20 | <1 |
| Quick time (s) | 11.4 | 11–18 |
| International normalized ratio | 1.01 | 1–2 |
| D-dimers (ng/mL) | 2300 | <250 |
| Laboratory Test | Day 11 of Admission | Day 16 of Admission |
|---|---|---|
| White blood cells count (×103/mm3) | 9.4 | 7.8 |
| Neutrophils (%) | 58 | 61 |
| Bands (%) | – | – |
| Thrombocytes (×103/mm3) | 521 | 417 |
| Erythrocyte sedimentation rate (mm/1 h) | 66 | 59 |
| Fibrinogen (mg/dL) | 266 | – |
| C-reactive protein (mg/L) | 12 | – |
| Procalcitonin (ng/mL, semiquantitative) | <1 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giubelan, L.; Dragonu, L.; Pădureanu, V.; Neacșu, A.; Mănescu, M.; Stoian, A.C.; Dumitrescu, F. Lemierre’s Syndrome: Case Presentation of a Rare and Possibly Life-Threatening Condition. Medicina 2021, 57, 1102. https://doi.org/10.3390/medicina57101102
Giubelan L, Dragonu L, Pădureanu V, Neacșu A, Mănescu M, Stoian AC, Dumitrescu F. Lemierre’s Syndrome: Case Presentation of a Rare and Possibly Life-Threatening Condition. Medicina. 2021; 57(10):1102. https://doi.org/10.3390/medicina57101102
Chicago/Turabian StyleGiubelan, Lucian, Livia Dragonu, Vlad Pădureanu, Alexandru Neacșu, Mirela Mănescu, Andreea Cristina Stoian, and Florentina Dumitrescu. 2021. "Lemierre’s Syndrome: Case Presentation of a Rare and Possibly Life-Threatening Condition" Medicina 57, no. 10: 1102. https://doi.org/10.3390/medicina57101102
APA StyleGiubelan, L., Dragonu, L., Pădureanu, V., Neacșu, A., Mănescu, M., Stoian, A. C., & Dumitrescu, F. (2021). Lemierre’s Syndrome: Case Presentation of a Rare and Possibly Life-Threatening Condition. Medicina, 57(10), 1102. https://doi.org/10.3390/medicina57101102







