Polymorphism in Gene for ABCC2 Transporter Predicts Methotrexate Drug Survival in Patients with Psoriasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Determinants of Drug Survival
2.3. DNA Extraction and Genotyping
2.4. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Change in Prescription Practice: Influence of Time of Methotrexate Introduction
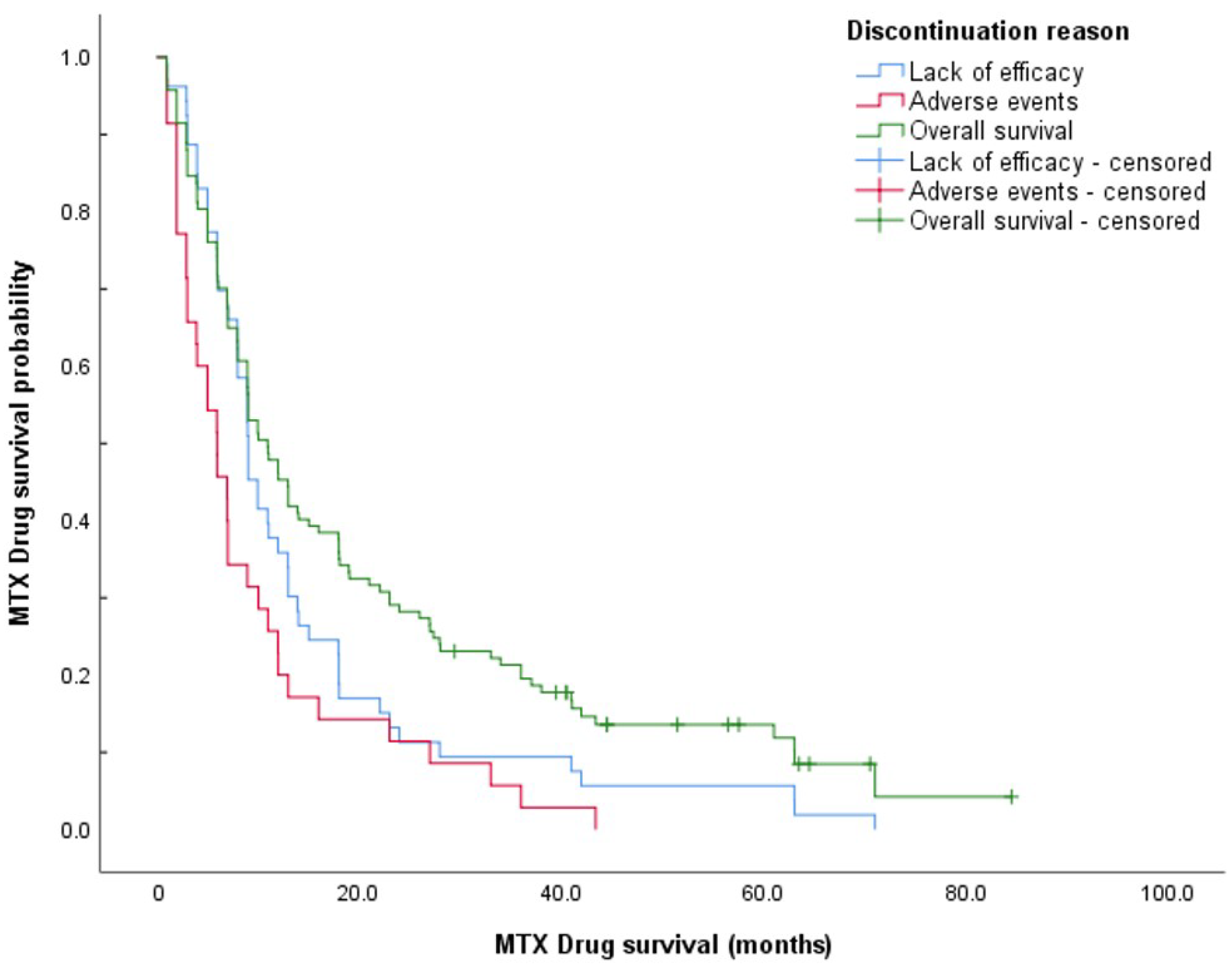
3.3. Methotrexate Drug Survival in Patients Starting Therapy in 2010 or Later
3.4. Predictors of Drug Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaushik, S.B.; Lebwohl, M.G. Review of safety and efficacy of approved systemic psoriasis therapies. Int. J. Dermatol. 2019, 58, 649–658. [Google Scholar] [CrossRef]
- Yélamos, O.; Puig, L. Systemic methotrexate for the treatment of psoriasis. Expert Rev. Clin. Immunol. 2015, 11, 553–563. [Google Scholar] [CrossRef]
- Menter, A.; Gelfand, J.M.; Connor, C.; Armstrong, A.W.; Cordoro, K.M.; Davis, D.M.; Elewski, B.E.; Gordon, K.B.; Gottlieb, A.B.; Kaplan, D.H.; et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J. Am. Acad. Dermatol. 2020, 82, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; Boonen, E.; De Jong, I.; Garcia-Doval, P.; Gisondi, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 1: Treatment and monitoring recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef] [PubMed]
- Zweegers, J.; Otero, M.E.; van ven Reek, J.M.P.A.; van Lümig, P.P.; Driessen, R.J.; Kievit, W.; Seyger, M.M.B.; van de Kerkhof, P.C.M.; de Jong, E.M.G. Effectiveness of biologic and conventional systemic therapies in adults with chronic plaque psoriasis in daily practice: A systematic review. Acta Derm. Venereol. 2016, 96, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Doval, I.; Carretero, G.; Vanaclocha, F.; Ferrandiz, C.; Daudén, E.; Sánchez-Carazo, J.L.; Alsina, M.; Herrera-Ceballos, E.; Gómez-García, F.-J.; Ferrán, M.; et al. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: Patients ineligible vs eligible for randomized controlled trials. Arch. Dermatol. 2012, 148, 463–470. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Ogston, S.; Foerster, J. Safety and efficacy of methotrexate in psoriasis: A meta-analysis of published trials. PLoS ONE 2016, 11, e0153740. [Google Scholar] [CrossRef]
- Van den Reek, J.M.P.A.; Kievit, W.; Gniadecki, R.; Goeman, J.J.; Zweegers, J.; van de Kerkhof, P.C.M.; Seyger, M.M.B.; de Jong, E.M.G.J. Drug survival studies in dermatology: Principles, purposes, and pitfalls. J. Investig. Dermatol. 2015, 135, 1–5. [Google Scholar] [CrossRef]
- Mourad, A.; Straube, S.; Armijo-Olivo, S.; Gniadecki, R. Factors predicting persistence of biologic drugs in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2019, 181, 450–458. [Google Scholar] [CrossRef]
- Arnold, T.; Schaarschmidt, M.L.; Herr, R.; Fischer, J.E.; Goerdt, S.; Peitsch, W.K. Drug survival rates and reasons for drug discontinuation in psoriasis. J. Dtsch. Dermatol. Ges. 2016, 14, 1089–1099. [Google Scholar] [CrossRef]
- Dávila-Seijo, P.; Dauden, E.; Carretero, G.; Ferrandiz, C.; Vanaclocha, F.; Gómez-García, F.J.; Herrera-Ceballos, E.; De la Cueva-Dobao, P.; Belinchón, I.; Sánchez-Carazo, J.-L.; et al. Survival of classic and biological systemic drugs in psoriasis: Results of the BIOBADADERM registry and critical analysis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1942–1950. [Google Scholar] [CrossRef]
- Puig, L.; Carrascosa, J.M.; Daudén, E.; Sulleiro, S.; Guisado, C. Drug survival of conventional systemic and biologic therapies for moderate-to-severe psoriasis in clinical practice in Spain: Prospective results from the SAHARA study. J. Dermatolog. Treat. 2020, 31, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Shalom, G.; Zisman, D.; Harman-Boehm, I.; Biterman, H.; Greenberg-Dotan, S.; Polishchuk, I.; Moser, H.; Freud, T.; Feldhamer, I.; Cohen, A.D. Factors associated with drug survival of methotrexate and acitretin in patients with psoriasis. Acta Derm. Venereol. 2015, 95, 973–977. [Google Scholar] [CrossRef][Green Version]
- Maul, J.-T.; Djamei, V.; Kolios, A.G.A.; Meier, B.; Czernielewski, J.; Jungo, P.; Yawalkar, N.; Mainetti, C.; Laffitte, E.; Spehr, C.; et al. Efficacy and survival of systemic psoriasis treatments: An analysis of the Swiss registry SDNTT. Dermatology 2016, 232, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Svedbom, A.; Ståhle, M. Real-world comparative effectiveness of adalimumab, etanercept and methotrexate: A Swedish register analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.E.; van den Reek, J.M.; Seyger, M.M.; van de Kerkhof, P.C.; Kievit, W.; de Jong, E.M. Determinants for drug survival of methotrexate in patients with psoriasis, split according to different reasons for discontinuation: Results of the prospective MTX-CAPTURE. Br. J. Dermatol. 2017, 177, 497–504. [Google Scholar] [CrossRef]
- Busger op Vollenbroek, F.T.M.; Doggen, C.J.M.; Janssens, R.W.A.; Bernelot Moens, H.J. Dermatological guidelines for monitoring methotrexate treatment reduce drug-survival compared to rheumatological guidelines. PLoS ONE 2018, 13, e0194401. [Google Scholar] [CrossRef]
- Akbulut Ozkok, T.; Topaloglu Demir, F.; Oguz Topal, I.; Kara Polat, A.; Karadag, A.S.; Aslan Kayiran, M.; Ozkur, E.; Altunay, I.K. Drug survival and predictor factors for discontinuation of methotrexate in psoriasis: A real-life multicenter study. Int. J. Dermatol. 2021, 60, 1140–1147. [Google Scholar] [CrossRef]
- Campalani, E.; Arenas, M.; Marinaki, A.M.; Lewis, C.M.; Barker, J.N.W.N.; Smith, C.H. Polymorphisms in folate, pyrimidine, and purine metabolism are associated with efficacy and toxicity of methotrexate in psoriasis. J. Investig. Dermatol. 2007, 127, 1860–1867. [Google Scholar] [CrossRef]
- Warren, R.B.; Smith, R.L.; Campalani, E.; Eyre, S.; Smith, C.H.; Barker, J.N.W.N.; Worthington, J.; Griffiths, C.E.M. Outcomes of methotrexate therapy for psoriasis and relationship to genetic polymorphisms. Br. J. Dermatol. 2009, 160, 438–441. [Google Scholar] [CrossRef]
- Warren, R.B.; Smith, R.L.L.; Campalani, E.; Eyre, S.; Smith, C.H.; Barker, J.N.W.N.; Worthington, J.; Griffiths, C.E.M. Genetic variation in efflux transporters influences outcome to methotrexate therapy in patients with psoriasis. J. Investig. Dermatol. 2008, 128, 1925–1929. [Google Scholar] [CrossRef]
- Chen, M.; Chen, W.; Liu, P.; Yan, K.; Lv, C.; Zhang, M.; Lu, Y.; Qin, Q.; Kuang, Y.; Zhu, W.; et al. The impacts of gene polymorphisms on methotrexate in Chinese psoriatic patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.H.; Lu, Y.; Yan, K.X.; Liu, P.P.; Chen, W.Q.; Shen, M.X.; He, Y.J.; Wu, L.S.; Qin, Q.S.; Zhou, X.C.; et al. Genetic polymorphism predicting Methotrexate efficacy in Chinese patients with psoriasis vulgaris. J. Dermatol. Sci. 2019, 93, 8–13. [Google Scholar] [CrossRef]
- Grželj, J.; Mlinarič-Raščan, I.; Marko, P.B.; Marovt, M.; Gmeiner, T.; Šmid, A. Polymorphisms in GNMT and DNMT3b are associated with methotrexate treatment outcome in plaque psoriasis. Biomed. Pharmacother. 2021, 138, 111456. [Google Scholar] [CrossRef] [PubMed]
- Shalom, G.; Cohen, A.D.; Feldhamer, I.; Comaneshter, D.; Freud, T.; Pavlovsky, L. Drug survival in patients with psoriasis is associated with the availability of biologic medications. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Sbidian, E.; Billionnet, C.; Weill, A.; Maura, G.; Mezzarobba, M. Persistence of apremilast in moderate-to-severe psoriasis: A real-world analysis of 14 147 apremilast- and methotrexate-naive patients in the French National Health Insurance database. Br. J. Dermatol. 2020, 182, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Cabello Zurita, C.; Grau Pérez, M.; Hernández Fernández, C.P.; González Quesada, A.; Valerón Almazán, P.; Vilar Alejo, J.; Carretero Hernández, G. Effectiveness and safety of Methotrexate in psoriasis: An eight-year experience with 218 patients. J. Dermatolog. Treat. 2017, 28, 401–405. [Google Scholar] [CrossRef]
- Dávila-Seijo, P.; García-Doval, I. Drug survival analysis is not a good method for assessing the safety or effectiveness of systemic therapies in psoriasis. Actas Dermo-Sifiliogr. 2017, 108, 3–5. [Google Scholar] [CrossRef]
- Pongparit, K.; Chularojanamontri, L.; Limphoka, P.; Silpa-Archa, N.; Wongpraparat, C. Effectiveness of and factors associated with clinical response to methotrexate under daily life conditions in Asian patients with psoriasis: A retrospective cohort study. J. Dermatol. 2018, 45, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Lie, E.; van der Heijde DDer Uhlig, T.; Heiberg, M.S.; Koldingsnes, W.; Rødevand, E.; Kaufmann, C.; Mikkelsen, K.; Kvien, T.K. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 671–676. [Google Scholar] [CrossRef]
- Jacobs, M.E.; Pouw, J.N.; Welsing, P.; Radstake, T.R.D.J.; Leijten, E.F.A. First-line csDMARD monotherapy drug retention in psoriatic arthritis: Methotrexate outperforms sulfasalazine. Rheumatology 2021, 60, 780–784. [Google Scholar] [CrossRef]
- Bruhn, O.; Cascorbi, I. Polymorphisms of the drug transporters ABCB1, ABCG2, ABCC2 and ABCC3 and their impact on drug bioavailability and clinical relevance. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1337–1354. [Google Scholar] [CrossRef]
- Zgheib, N.K.; Akra-Ismail, M.; Aridi, C.; Mahfouz, R.; Abboud, M.R.; Solh, H.; Muwakkit, S.A. Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharm. Genom. 2014, 24, 381–396. [Google Scholar] [CrossRef]
- Razali, R.H.; Noorizhab, M.N.F.; Jamari, H.; James, R.J.; Teh, K.H.; Ibrahim, H.M.; Teh, L.K.; Salleh, M. Association of ABCC2 with levels and toxicity of methotrexate in Malaysian Childhood Acute Lymphoblastic Leukemia (ALL). Pediatr. Hematol. Oncol. 2020, 37, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Rau, T.; Eerney, B.; Göres, R.; Eschenhagen, T.; Beck, J.; Langer, T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: Impact of ABCC2 polymorphisms on plasma concentrations. Clin. Pharmacol. Ther. 2006, 80, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, S.; Zimmermann, U.; Dazert, E.; Wruck, C.J.; Dazert, P.; Siegmund, W.; Kroemer, H.K.; Warzok, R.W.; Cascorbi, I. Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharm. J. 2007, 7, 56–65. [Google Scholar] [CrossRef]

| Gene | Variant | Wild-Type (n) | Heterozygous (n) | Homozygous (n) | Variant Allele Frequency (%) |
|---|---|---|---|---|---|
| BHMT | rs3733890 | 68 | 50 | 15 | 0.301 |
| GNMT | rs10948059 | 36 | 62 | 35 | 0.496 |
| DNMT3b | rs2424913 | 38 | 60 | 35 | 0.489 |
| ABCC2 | rs717620 | 95 | 30 | 7 | 0.167 |
| SLCO1B1 * | rs2306283 | 40 | 81 | 11 | 0.390 |
| SLCO1B1 * | rs4149056 | 84 | 45 | 2 | 0.187 |
| Characteristic | Patients Starting Treatment in 2010 or Later (n = 117) |
|---|---|
| Male (n (%)) | 71 (60.7) |
| Mean age at disease onset (years (SD)) | 29.2 (14.5) |
| Mean age at MTX introduction (years (SD) | 50.2 (12.9) |
| Early onset psoriasis 1 (n (%)) | 91 (77.8) |
| Positive family history (n (%)) | 60 (51.7) |
| Median disease duration at MTX introduction (years (range)) | 20.0 (0.00–66.0) |
| Median duration of MTX treatment (months (range) | 11.0 (0.99−84.5) |
| Discontinued treatment (n (%)) | 104 (88.9) |
| - Lack of efficacy | 53 (51.0) |
| - Adverse events | 35 (33.7) |
| - Other | 16 (15.4) |
| Factor | Detail | Patients (n) | Events (n (%Patients)) | Univariate Analysis (Log-Ranks) | Multivariate Analysis (Cox Regression) * | |
|---|---|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI) | p-Value | ||||
| Sex | Male (Ref) | 71 | 63 (88.7) | 0.487 | ||
| Female | 46 | 41 (89.1) | ||||
| Family history | Negative | 56 | 50 (89.3) | 0.592 | Not included | |
| Positive | 60 | 53 (88.3) | ||||
| BHMT genotype | GG (Ref] | 57 | 49 (86.0) | 0.203 | ||
| GA or AA | 60 | 55 (91.7) | ||||
| GNMT genotype | CC (Ref) | 30 | 25 (83.3) | 0.750 | ||
| CT or TT | 87 | 79 (90.8) | ||||
| DNMT3b genotype | CC (Ref] | 34 | 28 (82.4) | 0.630 | ||
| CT or TT | 83 | 76 (91.6) | ||||
| ABCC2 genotype | CC (Ref) | 86 | 80 (93.0) | 0.030 | 0.606 (0.380–0.967) | 0.036 |
| CT or TT | 30 | 23 (76.7) | ||||
| SLCO1B1 haplotype activity | High activity (Ref) | 74 | 67 (90.5) | 0.216 | ||
| Low activity | 41 | 35 (85.4) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grželj, J.; Marovt, M.; Marko, P.B.; Mlinarič-Raščan, I.; Gmeiner, T.; Šmid, A. Polymorphism in Gene for ABCC2 Transporter Predicts Methotrexate Drug Survival in Patients with Psoriasis. Medicina 2021, 57, 1050. https://doi.org/10.3390/medicina57101050
Grželj J, Marovt M, Marko PB, Mlinarič-Raščan I, Gmeiner T, Šmid A. Polymorphism in Gene for ABCC2 Transporter Predicts Methotrexate Drug Survival in Patients with Psoriasis. Medicina. 2021; 57(10):1050. https://doi.org/10.3390/medicina57101050
Chicago/Turabian StyleGrželj, Jasna, Maruška Marovt, Pij B. Marko, Irena Mlinarič-Raščan, Tanja Gmeiner, and Alenka Šmid. 2021. "Polymorphism in Gene for ABCC2 Transporter Predicts Methotrexate Drug Survival in Patients with Psoriasis" Medicina 57, no. 10: 1050. https://doi.org/10.3390/medicina57101050
APA StyleGrželj, J., Marovt, M., Marko, P. B., Mlinarič-Raščan, I., Gmeiner, T., & Šmid, A. (2021). Polymorphism in Gene for ABCC2 Transporter Predicts Methotrexate Drug Survival in Patients with Psoriasis. Medicina, 57(10), 1050. https://doi.org/10.3390/medicina57101050






