Phase Lag Entropy as a Surrogate Measurement of Hypnotic Depth during Sevoflurane Anesthesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Study Procedure
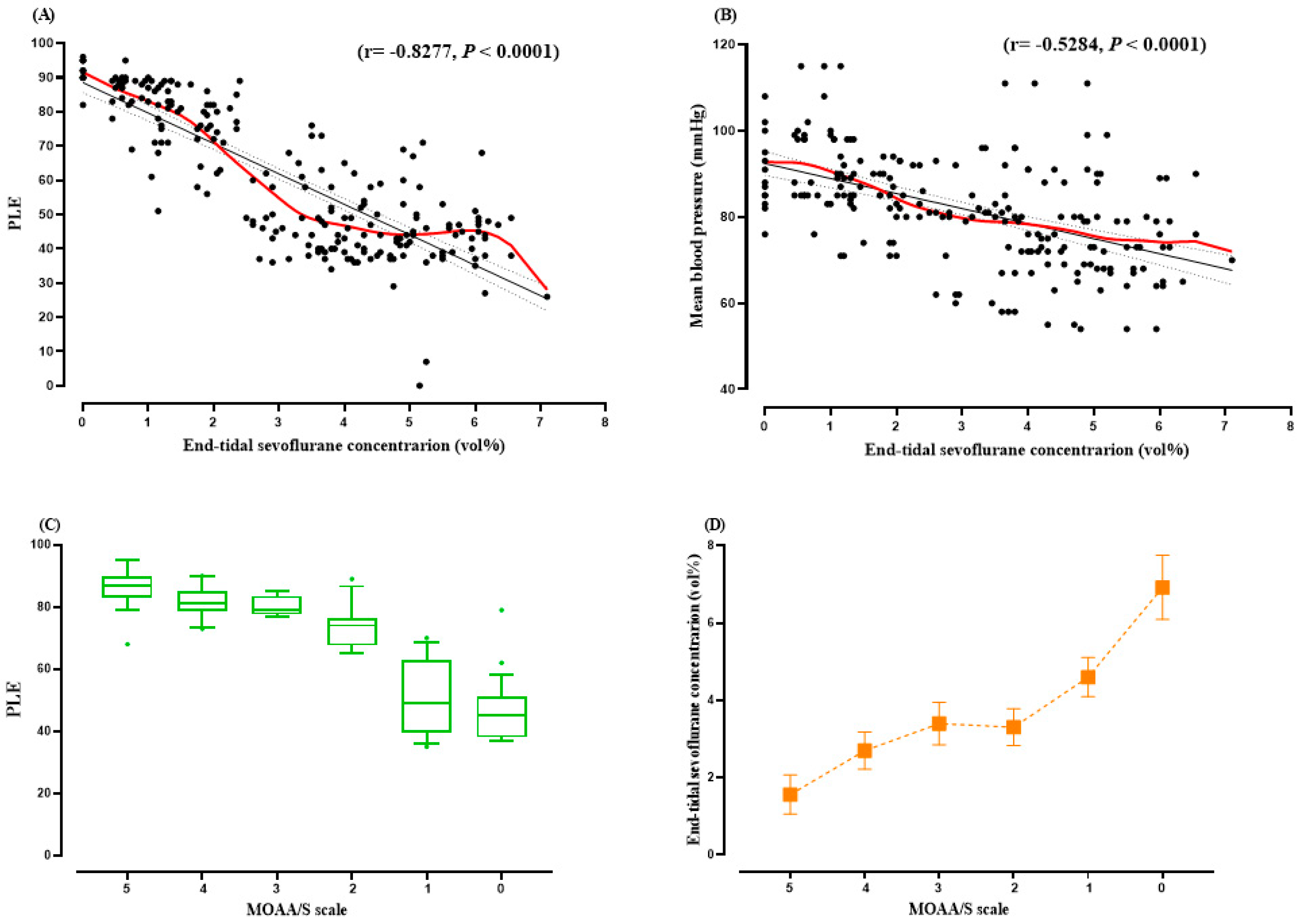
2.3. Correlation between Phase-Lag Entropy and End-Tidal Sevoflurane Concentration
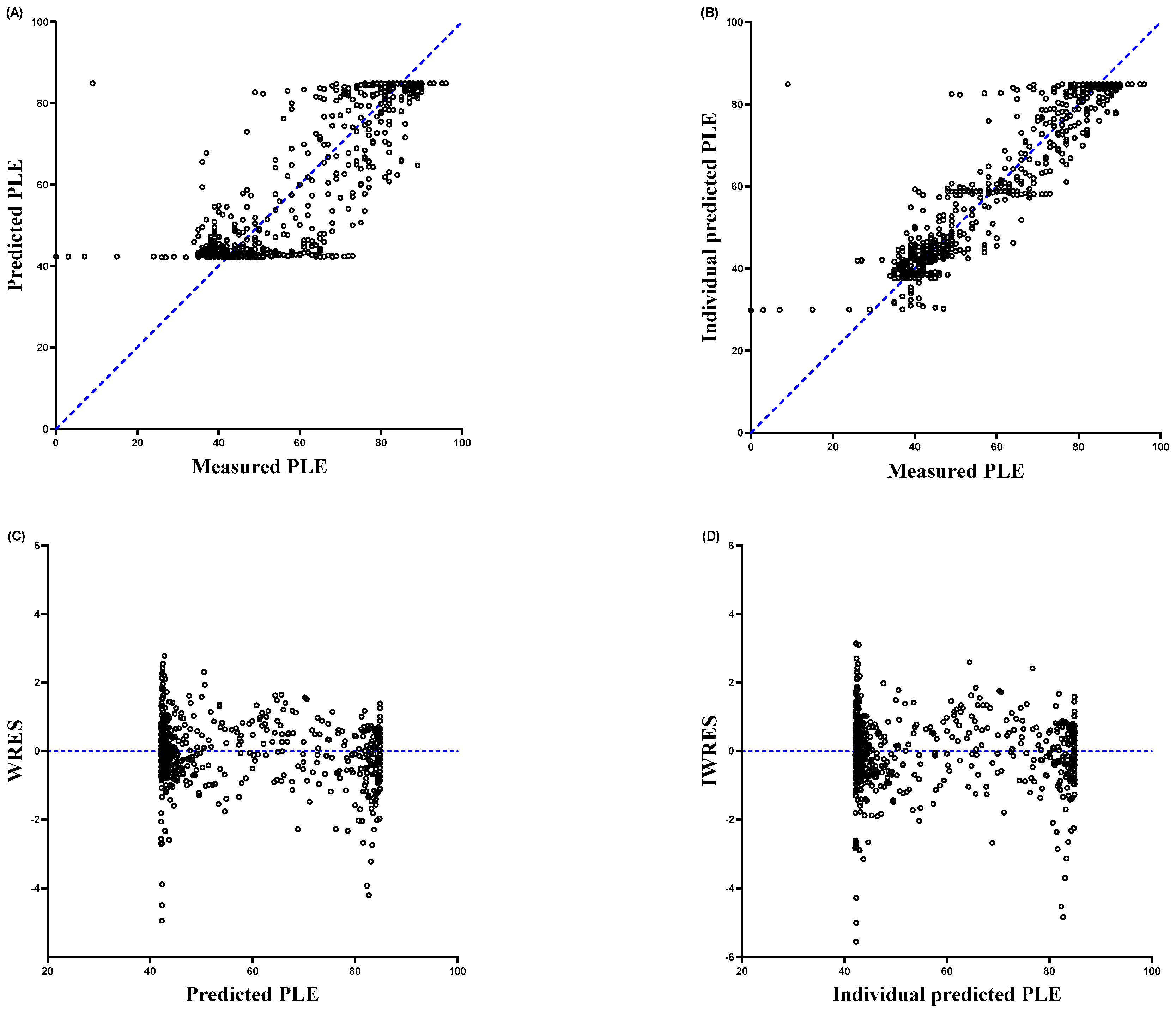
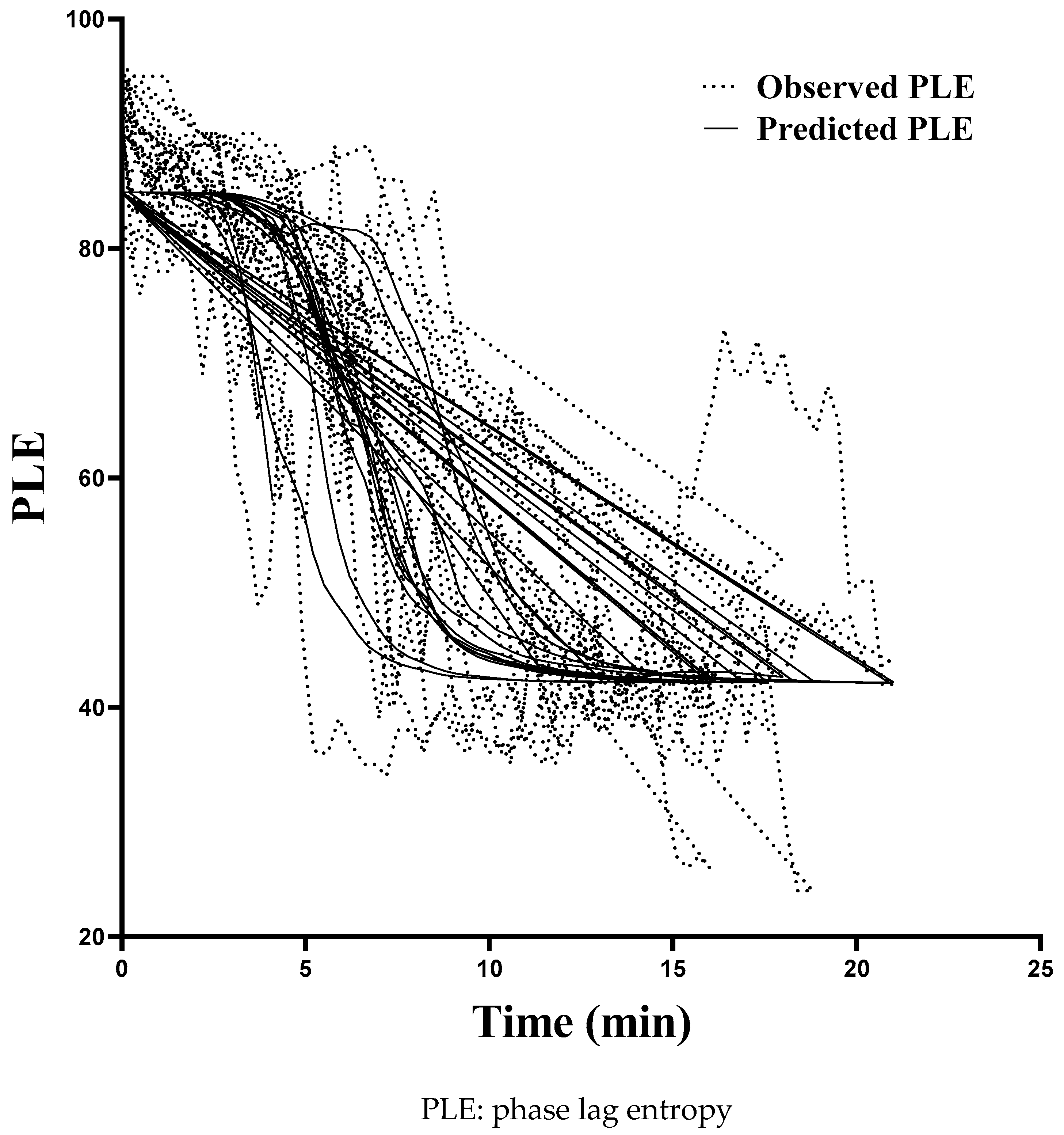
2.4. Pharmacodynamic Modeling
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, U.; Ku, S.; Noh, G.; Baek, S.; Choi, B.; Mashour, G.A. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology 2013, 118, 1264–1275. [Google Scholar] [CrossRef] [Green Version]
- Alkire, M.T.; Hudetz, A.G.; Tononi, G. Consciousness and Anesthesia. Science 2008, 322, 876–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehaene, S.; Changeux, J.-P. Experimental and Theoretical Approaches to Conscious Processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akeju, O.; Westover, M.B.; Pavone, K.J.; Sampson, A.L.; Hartnack, K.E.; Brown, E.N.; Purdon, P.L. Effects of Sevoflurane and Propofol on Frontal Electroencephalogram Power and Coherence. Anesthesiology 2014, 121, 990–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oizumi, M.; Albantakis, L.; Tononi, G. From the phenomenology to the mechanisms of consciousness: Integrated Information Theory 3.0. PLoS Comput. Biol. 2014, 10, e1003588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahaba, A.A. Different Conditions That Could Result in the Bispectral Index Indicating an Incorrect Hypnotic State. Anesth. Analg. 2005, 101, 765–773. [Google Scholar] [CrossRef]
- Lee, H.; Noh, G.J.; Joo, P.; Choi, B.M.; Silverstein, B.H.; Kim, M.; Wang, J.; Jung, W.S.; Kim, S. Diversity of functional connectivity patterns is reduced in propofol-induced unconsciousness. Hum. Brain Mapp. 2017, 38, 4980–4995. [Google Scholar]
- Jun, M.R.; Yoo, J.H.; Park, S.Y.; Na, S.; Kwon, H.; Nho, J.-H.; Kim, S.I. Assessment of phase-lag entropy, a new measure of electroencephalographic signals, for propofol-induced sedation. Korean J. Anesthesiol. 2019, 72, 351–356. [Google Scholar] [CrossRef]
- Ki, S.; Kim, K.M.; Lee, Y.H.; Bang, J.Y.; Choi, B.M.; Noh, G.J. Phase lag entropy as a hypnotic depth indicator during propofol sedation. Anaesthesia 2019, 74, 1033–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K.H.; Kim, K.M.; Lee, S.K.; John, H.; Lee, J. Comparative Analysis of Phase Lag Entropy and Bispectral Index as Anesthetic Depth Indicators in Patients Undergoing Thyroid Surgery with Nerve Integrity Monitoring. J. Korean Med. Sci. 2019, 34, e151. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.E.; Kang, E.; Park, Y.H.; Lee, H.S.; Lee, S.J.; Shin, D.; Noh, G.J.; Lee, I.H.; Lee, K.H. Effect of depth of anesthesia on the phase lag entropy in patients undergoing general anesthesia by propofol: A STROBE-compliant study. Medicine 2020, 99, e21303. [Google Scholar] [CrossRef]
- Shin, H.W.; Kim, H.J.; Jang, Y.; You, H.S.; Huh, H.; Choi, Y.J.; Choi, S.U.; Hong, J.S. Monitoring of anesthetic depth and EEG band power using phase lag entropy during propofol anesthesia. BMC Anesthesiol. 2020, 20, 49. [Google Scholar] [CrossRef]
- Choi, B.M.; Yang, C.S.; Woo, D.H.; Koo, M.S.; Kim, S.H.; Lee, M.A.; Kwak, H.S.; Noh, G.J. Approximate entropy as the measurement of the electroencephalographic effect during sevoflurane induction. Korean J. Anesthesiol. 2008, 55, 404–411. [Google Scholar] [CrossRef]
- Vakkuri, A.; Jäntti, V.; Särkelä, M.; Lindgren, L.; Korttila, K.; Yli-Hankala, A. Epileptiform EEG during sevoflurane mask induction: Effect of delaying the onset of hyperventilation. Acta Anaesthesiol. Scand. 2000, 44, 713–719. [Google Scholar] [CrossRef]
- Chernik, D.A.; Gillings, D.; Laine, H.; Hendler, J.; Silver, J.M.; Davidson, A.B.; Schwam, E.M.; Siegel, J.L. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: Study with intravenous midazolam. J. Clin. Psychopharmacol. 1990, 10, 244–251. [Google Scholar]
- Schnider, T.W.; Minto, C.; Shafer, S.; Gambus, P.L.; Andresen, C.; Goodale, D.B.; Youngs, E.J. The Influence of Age on Propofol Pharmacodynamics. Anesthesiology 1999, 90, 1502–1516. [Google Scholar] [CrossRef]
- Smith, W.D.; Dutton, R.C.; Smith, T.N. Measuring the Performance of Anesthetic Depth Indicators. Anesthesiology 1996, 84, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Stasiowski, M.J.; Duława, A.; Król, S.; Marciniak, R.; Kaspera, W.; Niewiadomska, E.; Krawczyk, L.; Ładziński, P.; Grabarek, B.O.; Jałowiecki, P. Polyspikes and Rhythmic Polyspikes During Volatile Induction of General Anesthesia With Sevoflurane Result in Bispectral Index Variations. Clin. EEG Neurosci. 2020, 26, 1550059420974571. [Google Scholar]
- Stasiowski, M.; Duława, A.; Szumera, I.; Marciniak, R.; Niewiadomska, E.; Kaspera, W.; Krawczyk, L.; Ładziński, P.; Grabarek, B.O.; Jałowiecki, P. Variations in Values of State, Response Entropy and Haemodynamic Parameters Associated with Development of Different Epileptiform Patterns during Volatile Induction of General Anaesthesia with Two Different Anaesthetic Regimens Using Sevoflurane in Comparison with Intra-venous Induct: A Comparative Study. Brain Sci. 2020, 10, 366. [Google Scholar]
- Gambus, P.; Trocóniz, I.F. Pharmacokinetic-pharmacodynamic modelling in anaesthesia. Br. J. Clin. Pharmacol. 2014, 79, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Sheiner, L.B.; Stanski, D.R.; Vozeh, S.; Miller, R.D.; Ham, J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: Application to d-tubocurarine. Clin. Pharmacol. Ther. 1979, 25, 358–371. [Google Scholar] [CrossRef]
- Katoh, T.; Suzuki, A.; Ikeda, K. Electroencephalographic derivatives as a tool for predicting the depth of sedation and anesthesia induced by sevoflurane. Anesthesiology 1998, 88, 642–650. [Google Scholar] [CrossRef]
- Fahy, B.G.; Chau, D.F. The Technology of Processed Electroencephalogram Monitoring Devices for Assessment of Depth of Anesthesia. Anesth. Analg. 2018, 126, 111–117. [Google Scholar] [CrossRef]
- Soehle, M.; Ellerkmann, R.K.; Grube, M.; Kuech, M.; Wirz, S.; Hoeft, A.; Bruhn, J. Comparison between Bispectral Index and Patient State Index as Measures of the Electroencephalographic Effects of Sevoflurane. Anesthesiology 2008, 109, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, I.; Nathanson, M.; White, P.F. Sevoflurane-a long-awaited volatile anaesthetic. Br. J. Anaesth. 1996, 76, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Purdon, P.L.; Sampson, A.; Pavone, K.J.; Brown, E.N. Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures. Anesthesiology 2015, 123, 937–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudetz, A.G.; Liu, X.; Pillay, S. Dynamic repertoire of intrinsic brain states is reduced in propofolinduced unconsciousness. Brain Connect. 2015, 5, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barttfeld, P.; Uhrig, L.; Sitt, J.D.; Sigman, M.; Jarraya, B.; Dehaene, S. Signature of conscious-ness in the dynamics of resting-state brain activity. Proc. Natl. Acad. Sci. USA 2015, 112, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Bruhn, J.; Röpcke, H.; Hoeft, A. Approximate entropy as an electroencephalographic measure of anesthetic drug effect during desflurane anesthesia. Anesthesiology 2000, 92, 715–726. [Google Scholar] [CrossRef]
- Kreuzer, M.; Zanner, R.; Pilge, S.; Paprotny, S.; Kochs, E.F.; Schneider, G. Time delay of monitors of the hypnotic component of anesthesia: Analysis of state entropy and index of consciousness. Anesth. Analg. 2012, 115, 315–319. [Google Scholar] [CrossRef]

| Variables | |
|---|---|
| Age (years) | 50.00 (37.00–53.25) |
| Sex (male/female) | 8/5 (62%/38%) |
| Height (cm) | 166.50 (160.80–171.28) |
| Weight (kg) | 66.10 (62.75–73.83) |
| ASA classification (Ⅰ/Ⅱ) | 8/5 (62%/38%) |
| BMI (kg/m2) | 25.51 (23.46–26.24) |
| Anesthesia time (min) | 185.00 (116.25–202.50) |
| Type of surgery | |
| Neck mass removal | 3 (23%) |
| Tympanoplasty | 8 (62%) |
| Parotid tumor removal | 2 (15%) |
| Parameter | Estimates (RSE, %) | CV (%) | Median (2.5–97.5%) |
|---|---|---|---|
| E0 | 84.9 (0.79) | ‒ | 85.0 (82.9–86.8) |
| Emax | 42 (5.76) | 18.73 | 41.6 (38.7–45) |
| Ce50,vol% | 1.81 (8.95) | 27.82 | 1.80 (1.8–2.1) |
| γ | 4.78 (10.61) | ‒ | 4.92 (4.5–5.5) |
| ke0 | 0.692 (33.82) | 67.68 | 0.673 (0.42–0.80) |
| 2 | 1 | ‒ | ‒ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-M.; Lee, K.-H.; Park, J.-H. Phase Lag Entropy as a Surrogate Measurement of Hypnotic Depth during Sevoflurane Anesthesia. Medicina 2021, 57, 1034. https://doi.org/10.3390/medicina57101034
Kim K-M, Lee K-H, Park J-H. Phase Lag Entropy as a Surrogate Measurement of Hypnotic Depth during Sevoflurane Anesthesia. Medicina. 2021; 57(10):1034. https://doi.org/10.3390/medicina57101034
Chicago/Turabian StyleKim, Kyung-Mi, Ki-Hwa Lee, and Jae-Hong Park. 2021. "Phase Lag Entropy as a Surrogate Measurement of Hypnotic Depth during Sevoflurane Anesthesia" Medicina 57, no. 10: 1034. https://doi.org/10.3390/medicina57101034
APA StyleKim, K.-M., Lee, K.-H., & Park, J.-H. (2021). Phase Lag Entropy as a Surrogate Measurement of Hypnotic Depth during Sevoflurane Anesthesia. Medicina, 57(10), 1034. https://doi.org/10.3390/medicina57101034






