The Optimizing Background Infusion Mode Decreases Intravenous Patient-Controlled Analgesic Volume and Opioid Consumption Compared to Fixed-Rate Background Infusion in Patients Undergoing Laparoscopic Cholecystectomy: A Prospective, Randomized, Controlled, Double-Blind Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Statement
2.2. Selection of Study Population
2.3. Randomization and Masking
2.4. Interventions
2.5. Outcomes
2.6. Sample Size
2.7. Analysis
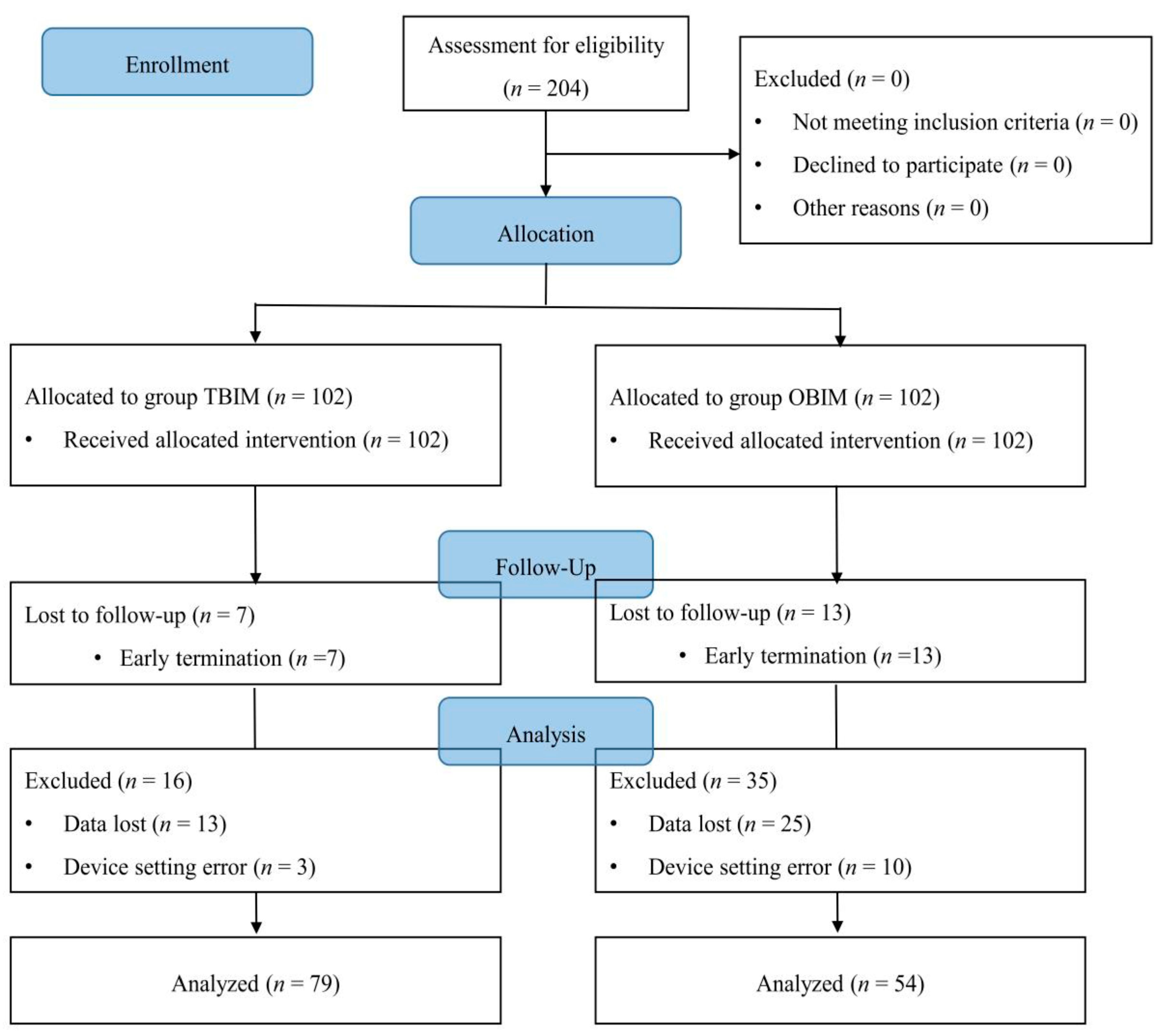
3. Results
3.1. Demographic Data
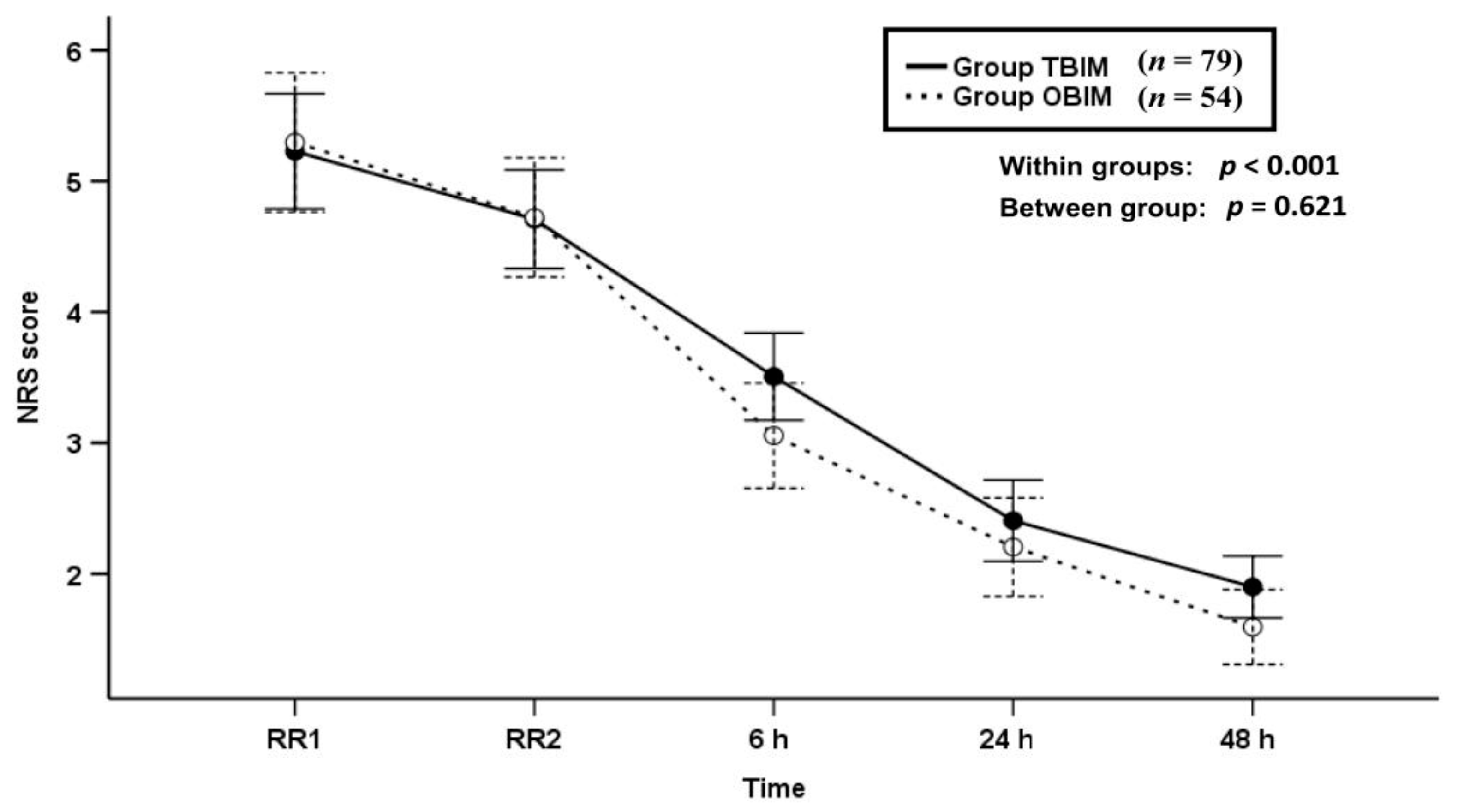
3.2. NRS Scores
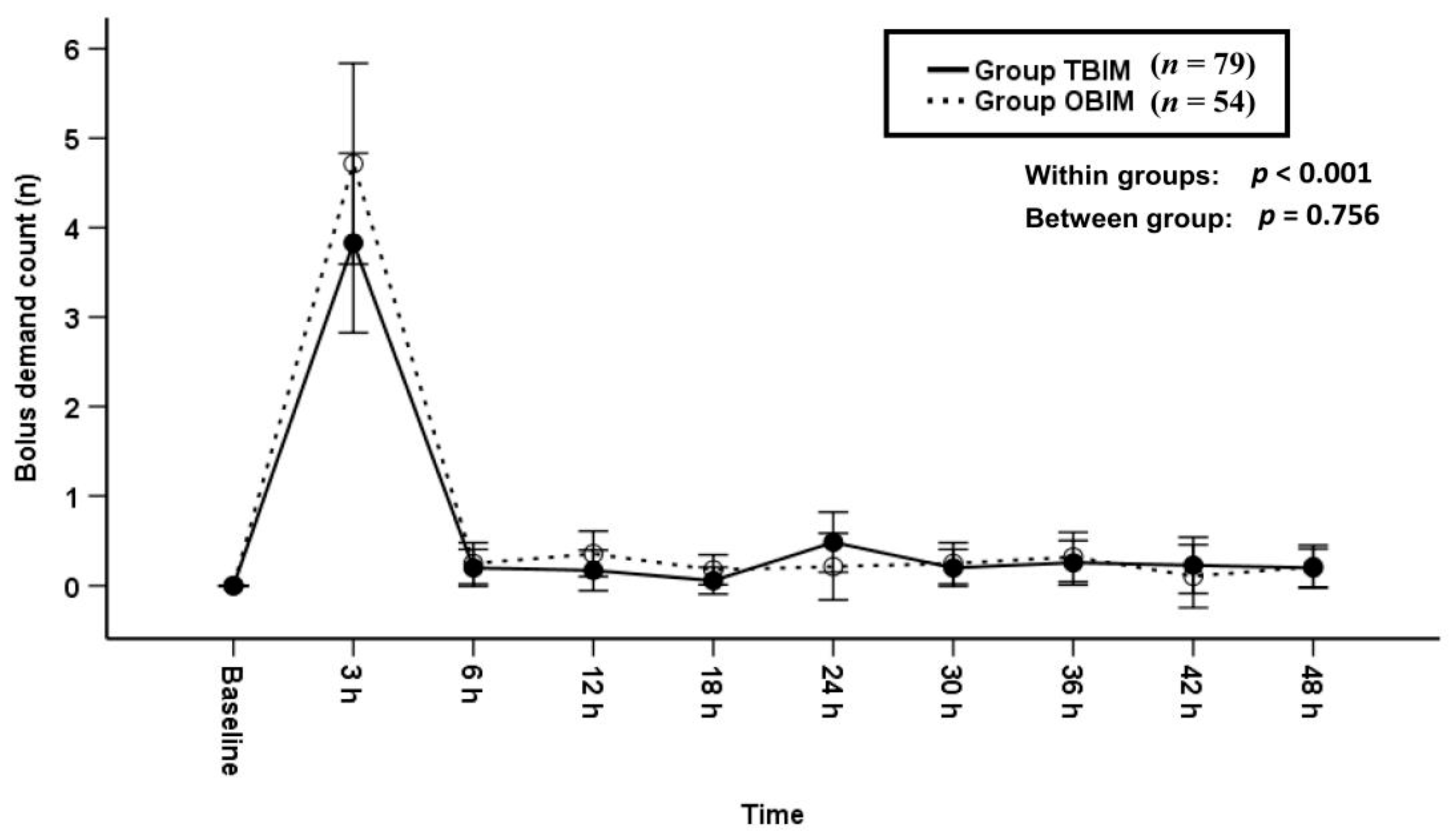
3.3. Bolus Demand Counts
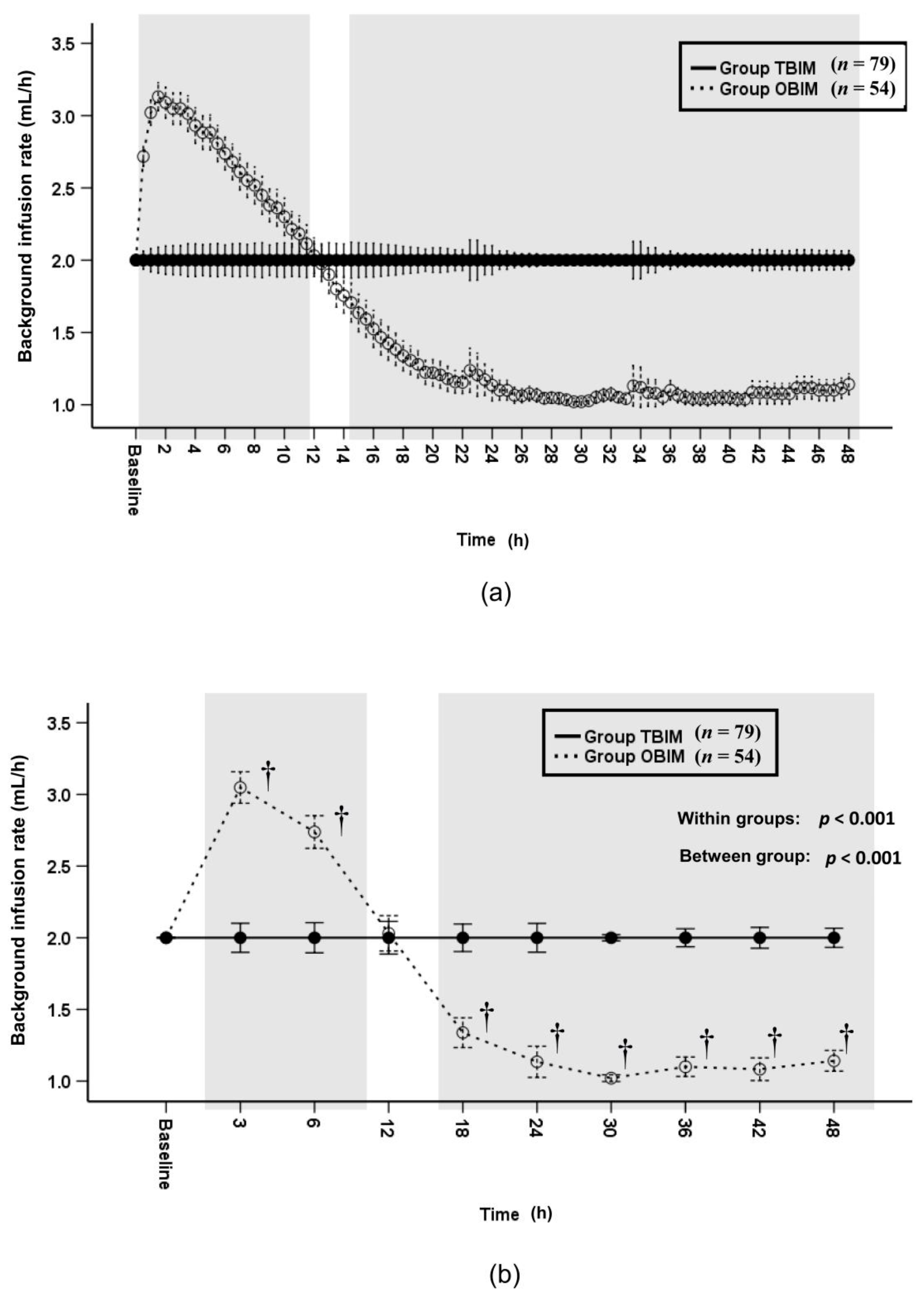
3.4. Background Infusion Rate
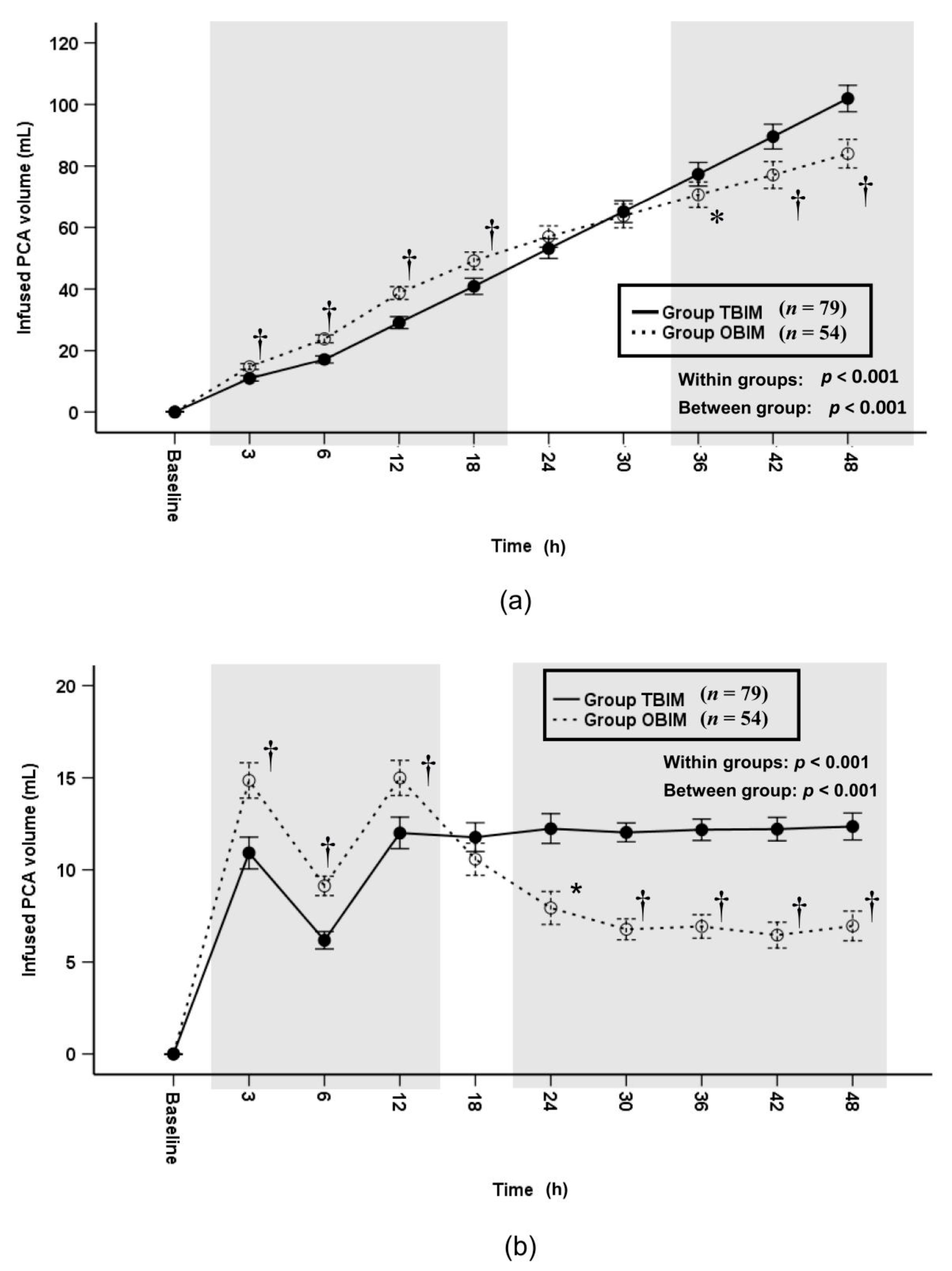
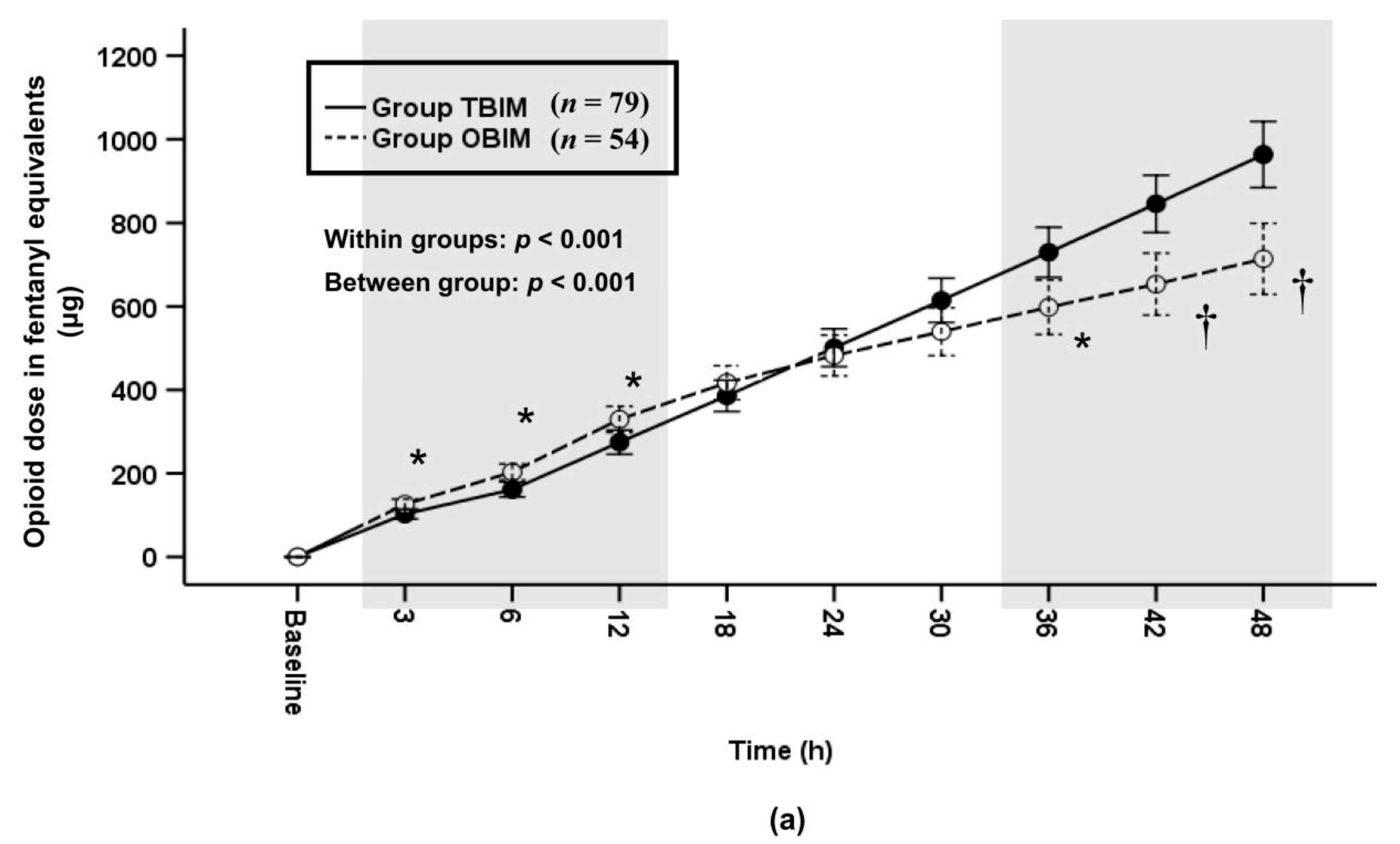
3.5. Infused PCA Volumes and Infused Opioid Doses
3.6. Rescue Drugs and Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grass, J.A. Patient-controlled analgesia. Anesth. Analg. 2005, 101, S44–S61. [Google Scholar] [CrossRef]
- Gepstein, R.; Arinzon, Z.; Folman, Y.; Shuval, I.; Shabat, S. Efficacy and complications of patient-controlled analgesia treatment after spinal surgery. Surg. Neurol. 2007, 67, 360–366. [Google Scholar] [CrossRef] [PubMed]
- McNicol, E.D.; Ferguson, M.C.; Hudcova, J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kye-Min, K. Analysis of the current state of postoperative patient-controlled analgesia in Korea. Anesthesiol. Pain Med. 2016, 11, 28–35. [Google Scholar]
- Lehmann, K.A. Recent developments in patient-controlled analgesia. J. Pain Symptom Manag. 2005, 29, S72–S89. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.V.; Fomsgaard, J.S.; Dahl, J.B.; Mathiesen, O. Insufficient pain management after spine surgery. Dan. Med. J. 2014, 61, A4835. [Google Scholar] [PubMed]
- Parker, R.K.; Holtmann, B.; White, P.F. Effects of a nighttime opioid infusion with PCA therapy on patient comfort and analgesic requirements after abdominal hysterectomy. Anesthesiology 1992, 76, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Smythe, M.A.; Zak, M.B.; O’Donnell, M.P.; Schad, R.F.; Dmuchowski, C.F. Patient-controlled analgesia versus patient-controlled analgesia plus continuous infusion after hip replacement surgery. Ann. Pharmacother. 1996, 30, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Liu, K.; Tan, P.H.; Chia, Y.Y. Effects of postoperative background PCA morphine infusion on pain management and related side effects in patients undergoing abdominal hysterectomy. J. Clin. Anesth. 2011, 23, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Baek, C.W.; Kang, H.; Park, Y.H.; Choi, G.J.; Jung, Y.H.; Woo, Y.C. A comparison of 2 intravenous patient-controlled analgesia modes after spinal fusion surgery: Constant-rate background infusion versus variable-rate feedback infusion, a randomized controlled trial. Medicine 2019, 98, e14753. [Google Scholar] [CrossRef] [PubMed]
- Sng, B.L.; Zhang, Q.; Leong, W.L.; Ocampo, C.; Assam, P.N.; Sia, A.T. Incidence and characteristics of breakthrough pain in parturients using computer-integrated patient-controlled epidural analgesia. J. Clin. Anesth. 2015, 27, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Yoon, S.H.; Kim, J.Y.; Oh, C.H.; Jung, J.K.; Kim, J. Comparison of the Effects of Sufentanil and Fentanyl Intravenous Patient Controlled Analgesia after Lumbar Fusion. J. Korean Neurosurg. Soc. 2017, 60, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Sng, B.L.; Woo, D.; Leong, W.L.; Wang, H.; Assam, P.N.; Sia, A.T. Comparison of computer-integrated patient-controlled epidural analgesia with no initial basal infusion versus moderate basal infusion for labor and delivery: A randomized controlled trial. J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Sng, B.L.; Sia, A.T.; Lim, Y.; Woo, D.; Ocampo, C. Comparison of computer-integrated patient-controlled epidural analgesia and patient-controlled epidural analgesia with a basal infusion for labour and delivery. Anaesth. Intensive Care 2009, 37, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sia, A.T.; Lim, Y.; Ocampo, C.E. Computer-integrated patient-controlled epidural analgesia: A preliminary study on a novel approach of providing pain relief in labour. Singapore Med. J. 2006, 47, 951–956. [Google Scholar] [PubMed]
- Lim, Y.; Sia, A.T.; Ocampo, C.E. Comparison of computer integrated patient controlled epidural analgesia vs. conventional patient controlled epidural analgesia for pain relief in labour. Anaesthesia 2006, 61, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Min, S.K.; Chae, Y.J.; Lim, G.M.; Joe, H.B. Continuous Fentanyl Background Infusion Regimen Optimised by Patient-Controlled Analgesia for Acute Postoperative Pain Management: A Randomised Controlled Trial. J. Clin. Med. 2020, 9, 211. [Google Scholar] [CrossRef]
- Devin, C.J.; McGirt, M.J. Best evidence in multimodal pain management in spine surgery and means of assessing postoperative pain and functional outcomes. J. Clin. Neurosci. 2015, 22, 930–938. [Google Scholar] [CrossRef] [PubMed]

| Variables | Group TBIM (n = 102) | Group OBIM (n = 102) | p Value |
|---|---|---|---|
| Exclusion (No/Yes) | 79 (77.5)/23 (22.5) | 54 (52.9)/48 (47.1) | <0.001 |
| Causes for Exclusion | |||
| Data loss | 13 (12.7) | 25 (24.5) | |
| Early PCA termination | 7 (6.9) | 13 (12.7) | |
| Setting error | 3 (2.9) | 10 (9.8) | |
| Early PCA termination (No/Yes) | 95 (93.1)/7 (6.9) | 89 (87.3)/13 (12.7) | 0.214 |
| Causes for PCA termination | |||
| Nausea | 0 (0.0) | 2 (2.0) | |
| No pain | 7 (6.9) | 11 (10.8) |
| Variables | Group TBIM (n = 79) | Group OBIM (n = 54) | p Value |
|---|---|---|---|
| Age (y | 49.7 ± 12.3 | 49.1 ± 12.7 | 0.795 |
| Sex (M/F) | 39/40 | 34/20 | 0.122 |
| Height (cm) | 165.5 ± 8.4 | 166.3 ± 8.2 | 0.589 |
| Weight (kg) | 68.7 ± 13.6 | 68.4 ± 14.9 | 0.888 |
| ASA-PS (I/II/III) | 39/36/4 | 31/23/0 | 0.203 |
| Cumulative remifentanil (µg) | 397.9 ± 355.8 | 369.9 ± 303.9 | 0.638 |
| Operation time (min) | 45.8 ± 45.0 | 43.9 ± 33.5 | 0.789 |
| Anesthesia time (min) | 59.1 ± 46.5 | 54.2 ± 33.5 | 0.503 |
| Drugs | Group TBIM (n = 79) | Group OBIM (n = 54) | p Value |
|---|---|---|---|
| Fentanyl (μg) | 1195.5 ± 180.7 (n =44) | 1276.5 ± 172.4 (n = 23)) | 0.081 |
| Sufentanil (μg) | 181.8 ± 34.8 (n = 35) | 169.1 ± 35.9 (n = 31) | 0.149 |
| Nefopam (mg) | 160.0 ± 0.0 (160.0–160.0) | 160.0 ± 0.0 | 1.000 |
| Ramosetron (mg) | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.000 |
| Variables | Groups | Time | Total | |||
|---|---|---|---|---|---|---|
| RR2 | 6 h | 24 h | 48 h | |||
| Analgesics | Group TBIM (n = 79) | 1 (1.3) | 14 (17.7) | 8 (10.1) | 6 (7.6) | 23 (29.1) |
| Group OBIM (n = 54) | 1 (1.9) | 8 (14.8) | 4 (7.4) | 1 (1.9) | 10 (18.5) | |
| p value | 1.000 | 0.658 | 0.761 | 0.240 | 0.165 | |
| Antiemetics | Group TBIM (n = 79) | 0 (0) | 0 (0) | 3 (3.8) | 0 (0) | 3 (3.8) |
| Group OBIM (n = 54) | 0 (0) | 2 (3.7) | 1 (1.9) | 1 (1.9) | 3 (5.6) | |
| p value | - | 0.163 | 0.646 | 0.406 | 0.686 | |
| Variables | Groups | Time | ||||
|---|---|---|---|---|---|---|
| RR2 | 6 h | 24 h | 48 h | |||
| Analgesics | Group TBIM (n = 79) | Tramadol | 1 (1.3) | 14 (17.7) | 7 (8.9) | 6 (7.6) |
| Diclofenac | 0 (0) | 1 (1.3) | 0 (0) | 0 (0) | ||
| Fentanyl | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) | ||
| Group OBIM (n = 54) | Tramadol | 1 (1.9) | 6 (11.1) | 8 (10.1) | 1 (1.9) | |
| Diclofenac | 0 (0) | 1 (1.9) | 4 (7.4) | 0 (0) | ||
| Fentanyl | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| p value | 1.000 | 0.564 | 0.673 | 0.240 | ||
| Cumulative opioid dose in fentanyl equivalents (μg) | Cumulative diclofenac dose (mg) | |||||
| Group TBIM (n = 79) | 31.5 (16.3−46.7) | 1.1 (−1.1 to 3.4) | ||||
| Group OBIM (n = 54) | 20.7 (5.2−36.3) | 1.7 (−1.7 to 5.0) | ||||
| p value | 0.339 | 0.787 | ||||
| Mean difference (95% CI) | 10.8 (−11.4 to 33.0) | −0.5 (−4.4 to 3.3) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, K.T.; So, K.Y.; Kim, S.U.; Kim, S.H. The Optimizing Background Infusion Mode Decreases Intravenous Patient-Controlled Analgesic Volume and Opioid Consumption Compared to Fixed-Rate Background Infusion in Patients Undergoing Laparoscopic Cholecystectomy: A Prospective, Randomized, Controlled, Double-Blind Study. Medicina 2021, 57, 42. https://doi.org/10.3390/medicina57010042
Jung KT, So KY, Kim SU, Kim SH. The Optimizing Background Infusion Mode Decreases Intravenous Patient-Controlled Analgesic Volume and Opioid Consumption Compared to Fixed-Rate Background Infusion in Patients Undergoing Laparoscopic Cholecystectomy: A Prospective, Randomized, Controlled, Double-Blind Study. Medicina. 2021; 57(1):42. https://doi.org/10.3390/medicina57010042
Chicago/Turabian StyleJung, Ki Tae, Keum Young So, Seung Un Kim, and Sang Hun Kim. 2021. "The Optimizing Background Infusion Mode Decreases Intravenous Patient-Controlled Analgesic Volume and Opioid Consumption Compared to Fixed-Rate Background Infusion in Patients Undergoing Laparoscopic Cholecystectomy: A Prospective, Randomized, Controlled, Double-Blind Study" Medicina 57, no. 1: 42. https://doi.org/10.3390/medicina57010042
APA StyleJung, K. T., So, K. Y., Kim, S. U., & Kim, S. H. (2021). The Optimizing Background Infusion Mode Decreases Intravenous Patient-Controlled Analgesic Volume and Opioid Consumption Compared to Fixed-Rate Background Infusion in Patients Undergoing Laparoscopic Cholecystectomy: A Prospective, Randomized, Controlled, Double-Blind Study. Medicina, 57(1), 42. https://doi.org/10.3390/medicina57010042






