Feasibility of Ultra-Low-Dose CT for Bronchoscopy of Peripheral Lung Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT Protocol for Bronchoscopy of Peripheral Lung Lesions
2.3. CT Results and Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Objective Image Noise
3.2. Bronchial Wall Thickness and Wall-Area Ratio
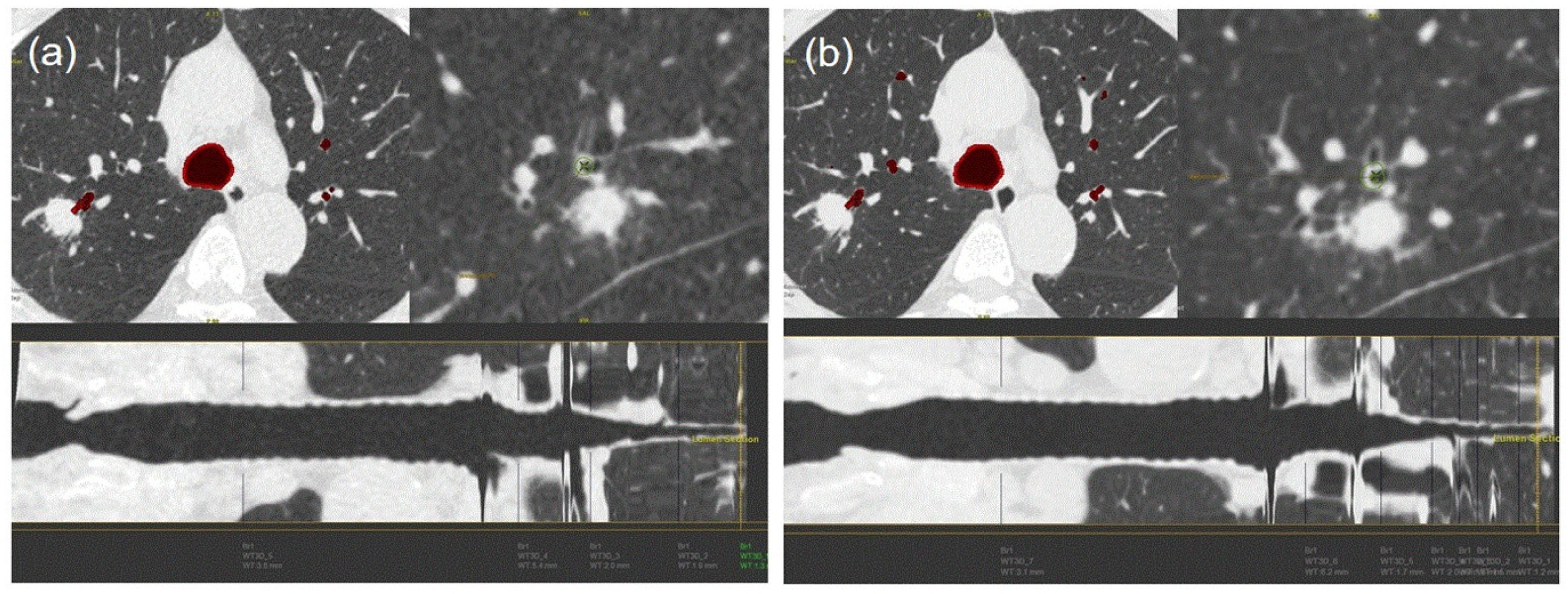
3.3. Bronchus Sign
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Lung Screening Trial Research Team; Church, T.R.; Black, W.C.; Aberle, D.R.; Berg, C.D.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; Gierada, D.S.; et al. Results of initial low-dose computed tomographic screening for lung cancer. N. Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar] [PubMed]
- Henschke, C.I.; Yankelevitz, D.F.; Mirtcheva, R.; McGuinness, G.; McCauley, D.; Miettinen, O.S.; ELCAP Group. CT screening for lung cancer: Frequency and significance of part-solid and nonsolid nodules. AJR Am. J. Roentgenol. 2002, 178, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Lewis, S.Z.; Diekemper, R.; Addrizzo-Harris, D.; Alberts, W.M. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, 7S–37S. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Hsiao, C.H.; Chang, Y.C.; Lee, J.M.; Shih, J.Y.; Wu, L.A.; Yu, C.J.; Liu, H.M.; Shih, T.T.F.; Yang, P.C. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J. Thorac. Oncol. 2012, 7, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Donington, J.; Lynch, W.R.; Mazzone, P.J.; Midthun, D.E.; Naidich, D.P.; Wiener, R.S. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e93S–e120S. [Google Scholar] [CrossRef]
- Kurimoto, N.; Miyazawa, T.; Okimasa, S.; Maeda, A.; Oiwa, H.; Miyazu, Y.; Murayama, M. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004, 126, 959–965. [Google Scholar] [CrossRef]
- Eom, J.S.; Mok, J.H.; Kim, I.; Lee, M.K.; Lee, G.; Park, H.; Lee, J.W.; Jeong, Y.J.; Kim, W.Y.; Jo, E.J.; et al. Radial probe endobronchial ultrasound using a guide sheath for peripheral lung lesions in beginners. BMC Pulm. Med. 2018, 18, 137. [Google Scholar] [CrossRef]
- Oki, M.; Saka, H.; Ando, M.; Asano, F.; Kurimoto, N.; Morita, K.; Kitagawa, C.; Kogure, Y.; Miyazawa, T. Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions. A randomized trial. Am. J. Respir. Crit. Care Med. 2015, 192, 468–476. [Google Scholar] [CrossRef]
- Ishida, T.; Asano, F.; Yamazaki, K.; Shinagawa, N.; Oizumi, S.; Moriya, H.; Munakata, M.; Nishimura, M.; Virtual Navigation in Japan Trial Group. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: A randomised trial. Thorax 2011, 66, 1072–1077. [Google Scholar] [CrossRef]
- Eberhardt, R.; Anantham, D.; Ernst, A.; Feller-Kopman, D.; Herth, F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2007, 176, 36–41. [Google Scholar] [CrossRef]
- McCollough, C.H.; Schueler, B.A. Calculation of effective dose. Med. Phys. 2000, 27, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T.; Willmann, J.K.; Hilfiker, P.R.; Weishaupt, D.; Seifert, B.; Crook, D.W.; Marincek, B.; Wildermuth, S. Thin-section CT of the lung: Does electrocardiographic triggering influence diagnosis? Radiology 2003, 229, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Meng, J.; Xie, X.; Zhang, H. Airway quantification using adaptive statistical iterative reconstruction-V on wide-detector low-dose CT: A validation study on lung specimen. Jpn. J. Radiol. 2019, 37, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Pandolfo, I.; Volta, S.; Russi, E.G.; Bartiromo, G.; Girone, G.; La Spada, F.; Barone, M.; Casablanca, G.; Minutoli, A. Bronchus sign on CT in peripheral carcinoma of the lung: Value in predicting results of transbronchial biopsy. AJR Am. J. Roentgenol. 1991, 157, 1181–1185. [Google Scholar] [CrossRef]
- Naidich, D.P.; Sussman, R.; Kutcher, W.L.; Aranda, C.P.; Garay, S.M.; Ettenger, N.A. Solitary pulmonary nodules. CT-bronchoscopic correlation. Chest 1988, 93, 595–598. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [PubMed]
- Pinsky, P.F.; Gierada, D.S.; Black, W.; Munden, R.; Nath, H.; Aberle, D.; Kazerooni, E. Performance of Lung-RADS in the National Lung Screening Trial: A retrospective assessment. Ann. Intern. Med. 2015, 162, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, G.; Kim, A.; Mok, J.; Lee, J.W.; Jeong, Y.J.; Jo, E.J.; Kim, M.H.; Lee, K.; Kim, K.U. Clinical outcomes of radial probe endobronchial ultrasound using a guide sheath for diagnosis of peripheral lung lesions in patients with pulmonary emphysema. Respir. Res. 2019, 20, 177. [Google Scholar] [CrossRef]
- Mehta, A.C.; Hood, K.L.; Schwarz, Y.; Solomon, S.B. The evolutional history of electromagnetic navigation bronchoscopy: State of the art. Chest 2018, 154, 935–947. [Google Scholar] [CrossRef]
- Chaddha, U.; Kovacs, S.P.; Manley, C.; Hogarth, D.K.; Cumbo-Nacheli, G.; Bhavani, S.V.; Kumar, R.; Shende, M.; Egan, J.P., 3rd; Murgu, S.; et al. Robot-assisted bronchoscopy for pulmonary lesion diagnosis: Results from the initial multicenter experience. BMC Pulm. Med. 2019, 19, 243. [Google Scholar] [CrossRef]
- Kemp, S.V. Navigation Bronchoscopy. Respiration 2020, 99, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Asano, F.; Matsuno, Y.; Shinagawa, N.; Yamazaki, K.; Suzuki, T.; Ishida, T.; Moriya, H. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest 2006, 130, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, D.P.; Khor, Y.H.; Manser, R.L.; Irving, L.B. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: Systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Wang Memoli, J.S.; Nietert, P.J.; Silvestri, G.A. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012, 142, 385–393. [Google Scholar] [CrossRef]
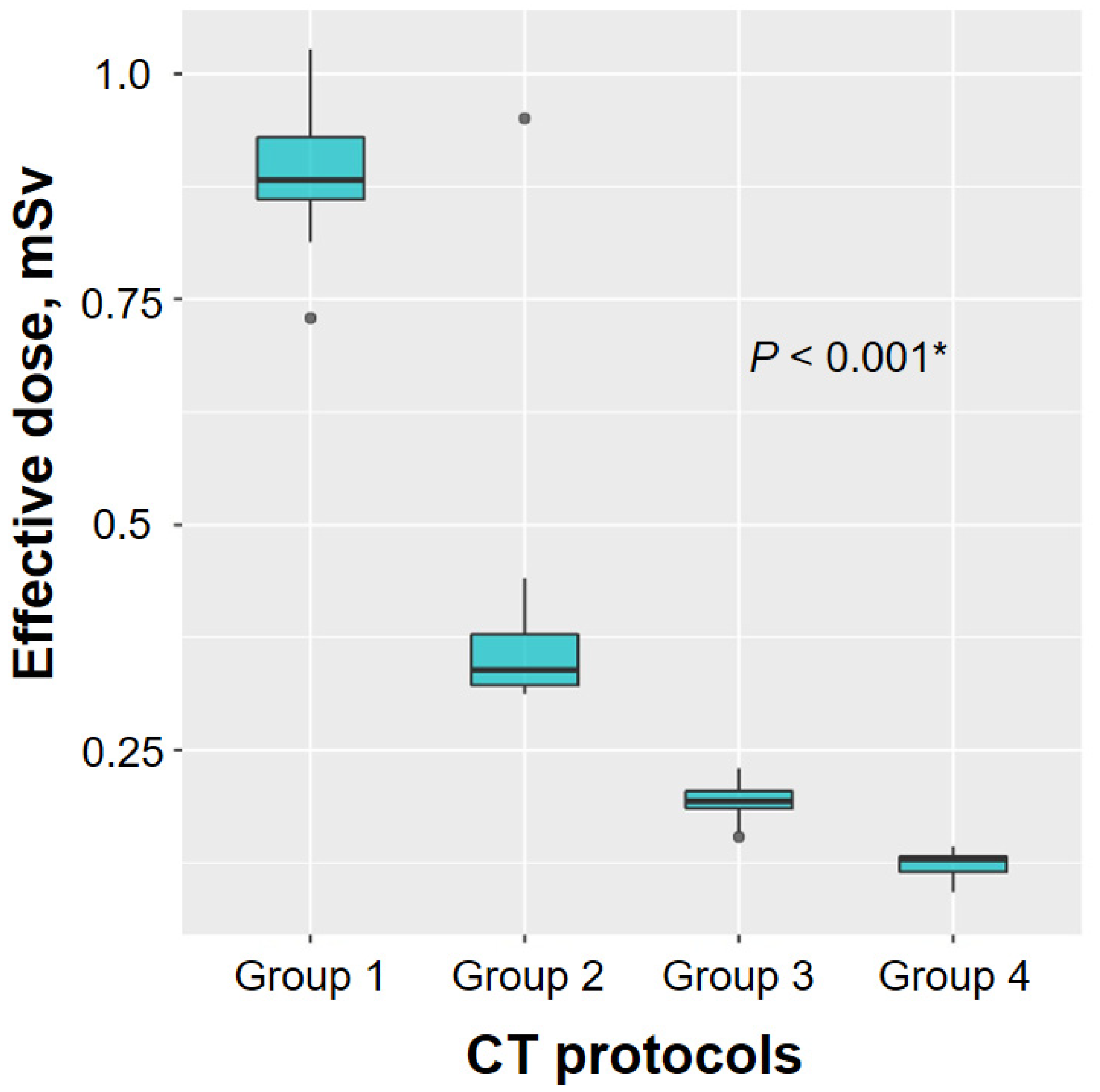

| Group 1 (n = 25) | Group 2 (n = 20) | Group 3 (n = 24) | Group 4 (n = 22) | p-Value | |
|---|---|---|---|---|---|
| Radiologist 1 | |||||
| Image Noise on SCT | 19 (17–20) | 19 (18–22) | 19 (17–21) | 19 (18–22) | 0.690 |
| Image Noise on Ultra-LDCT | 25 (24–27) | 29 (28–32) | 33 (32–34) | 38 (36–46) | <0.001 |
| Difference of Image Noise | 6 (4–8) | 11 (9–13) | 14 (12–17) | 18 (17–22) | <0.001 |
| Radiologist 2 | |||||
| Image Noise on SCT | 20 (17–21) | 20 (17–21) | 19 (18–21) | 18 (17–22) | 0.990 |
| Image Noise on Ultra-LDCT | 27 (23–29) | 31 (28–34) | 34 (32–38) | 37 (34–41) | <0.001 |
| Difference of Image Noise | 8 (5–10) | 12 (10–14) | 16 (13–19) | 18 (14–21) | <0.001 |
| Group 1 (n = 25) | Group 2 (n = 20) | Group 3 (n = 24) | Group 4 (n = 22) | p-Value | |
|---|---|---|---|---|---|
| Radiologist 1 | |||||
| WT on SCT, mm | 1.8 (1.3–2.6) | 1.6 (1.3–2.0) | 1.5 (1.2–2.2) | 1.7 (1.3–2.2) | 0.649 |
| WT on ultra-LDCT, mm | 1.7 (1.4–2.3) | 1.7 (1.5–2.2) | 2.0 (1.7–2.4) | 2.5 (2.0–3.1) | 0.018 |
| Difference of WT | 0.5 (0.3–0.9) | 0.5 (0.3–0.7) | 0.5 (0.3–0.6) | 0.9 (0.5–1.4) | 0.103 |
| Radiologist 2 | |||||
| WT on SCT, mm | 1.9 (1.3–2.6) | 1.7 (1.3–2.0) | 1.5 (1.2–2.1) | 1.6 (1.1–2.1) | 0.322 |
| WT on ultra-LDCT, mm | 1.7 (1.4–2.1) | 1.7 (1.4–1.9) | 1.9 (1.7–2.4) | 2.2 (1.7–3.1) | 0.009 |
| Difference of WT | 0.5 (0.2–1.0) | 0.5 (0.2–0.6) | 0.4 (0.2–0.5) | 0.8 (0.6–1.4) | 0.011 |
| Group 1 (n = 25) | Group 2 (n = 20) | Group 3 (n = 24) | Group 4 (n = 22) | p-Value | |
|---|---|---|---|---|---|
| Radiologist 1 | |||||
| WAR on SCT, % | 78 (73–84) | 77 (76–81) | 73 (71–79) | 76 (73–80) | 0.112 |
| WAR on ultra-LDCT, % | 79 (74–82) | 75 (73–80) | 77 (73–79) | 78 (73–82) | 0.505 |
| Difference of WAR | 5 (4–10) | 3 (1–5) | 4 (2–6) | 7 (3–11) | 0.058 |
| Radiologist 2 | |||||
| WAR on SCT, % | 79 (75–84) | 76 (75–81) | 75 (71–78) | 76 (73–81) | 0.166 |
| WAR on ultra-LDCT, % | 79 (75–81) | 77 (73–80) | 77 (72–79) | 78 (73–81) | 0.553 |
| Difference of WAR | 5 (2–10) | 4 (2–10) | 6 (3–9) | 10 (4–15) | 0.375 |
| Accuracy of Bronchus Sign on Ultra-LDCT | Group 1 (n = 25) | Group 2 (n = 20) | Group 3 (n = 24) | Group 4 (n = 22) | p-Value |
|---|---|---|---|---|---|
| Radiologist 1 | 25/25 (100) | 20/20 (100) | 20/24 (83) | 16/22 (73) | 0.003 |
| Radiologist 2 | 24/25 (96) | 20/20 (100) | 21/24 (88) | 18/22 (82) | 0.143 |
| Pulmonary Physician 1 | 25/25 (100) | 20/20 (100) | 23/24 (96) | 18/22 (82) | 0.017 |
| Pulmonary Physician 2 | 25/25 (100) | 19/20 (95) | 23/24 (96) | 17/22 (77) | 0.023 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, J.S.; Lee, G.; Roh, J.; Chung, H.S.; Jeong, Y.J. Feasibility of Ultra-Low-Dose CT for Bronchoscopy of Peripheral Lung Lesions. Medicina 2020, 56, 479. https://doi.org/10.3390/medicina56090479
Eom JS, Lee G, Roh J, Chung HS, Jeong YJ. Feasibility of Ultra-Low-Dose CT for Bronchoscopy of Peripheral Lung Lesions. Medicina. 2020; 56(9):479. https://doi.org/10.3390/medicina56090479
Chicago/Turabian StyleEom, Jung Seop, Geewon Lee, Jiyeon Roh, Hyun Sung Chung, and Yeon Joo Jeong. 2020. "Feasibility of Ultra-Low-Dose CT for Bronchoscopy of Peripheral Lung Lesions" Medicina 56, no. 9: 479. https://doi.org/10.3390/medicina56090479
APA StyleEom, J. S., Lee, G., Roh, J., Chung, H. S., & Jeong, Y. J. (2020). Feasibility of Ultra-Low-Dose CT for Bronchoscopy of Peripheral Lung Lesions. Medicina, 56(9), 479. https://doi.org/10.3390/medicina56090479





