Effects of Age on Long-Term Functional Recovery in Patients with Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessment
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Disclosures
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MBI | modified Barthel Index |
| mRS | modified Rankin Score |
| FAC | functional ambulation category |
| MMSE | Mini-Mental State Examination |
References
- Miller, E.L.; Murray, L.; Richards, L.; Zorowitz, R.D.; Bakas, T.; Clark, P.; Billinger, S.A.; on behalf of the American Heart Association Council on Cardiovascular Nursing and the Stroke Council. Comprehensive Overview of Nursing and Interdisciplinary Rehabilitation Care of the Stroke Patient. Stroke 2010, 41, 2402–2448. [Google Scholar] [CrossRef]
- Murakami, K.; Asayama, K.; Satoh, M.; Inoue, R.; Tsubota-Utsugi, M.; Hosaka, M.; Matsuda, A.; Nomura, K.; Murakami, T.; Kikuya, M.; et al. Risk Factors for Stroke among Young-Old and Old-Old Community-Dwelling Adults in Japan: The Ohasama Study. J. Atheroscler. Thromb. 2017, 24, 290–300. [Google Scholar] [CrossRef]
- Kwakkel, G.; Van Peppen, R.; Wagenaar, R.C.; Dauphinee, S.W.; Richards, C.; Ashburn, A.; Miller, K.; Lincoln, N.; Partridge, C.; Wellwood, I.; et al. Effects of Augmented Exercise Therapy Time After Stroke. Stroke 2004, 35, 2529–2539. [Google Scholar] [CrossRef]
- Dombovy, M.L.; Basford, J.R.; Whisnant, J.P.; Bergstralh, E.J. Disability and Use of Rehabilitation Services Following Stroke in Rochester, Minnesota, 1975–1979. Stroke 1987, 18, 830–836. [Google Scholar] [CrossRef]
- Bagg, S.; Pombo, A.P.; Hopman, W. Effect of age on functional outcomes after stroke rehabilitation. Stroke 2002, 33, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Counsell, C.; Dennis, M.; McDowall, M. Predicting functional outcome in acute stroke: Comparison of a simple six variable model with other predictive systems and informal clinical prediction. J. Neurol. Neurosurg. Psychiatry 2004, 75, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Appelros, P.; Stegmayr, B.; Terént, A. A review on sex differences in stroke treatment and outcome. Acta Neurol. Scand. 2009, 121, 359–369. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, K.-B.; Roh, H.; Ahn, M.-Y.; Hwang, H.-W. Gender Differences in the Functional Recovery after Acute Stroke. J. Clin. Neurol. 2010, 6, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, R.; Dahl, T.; Wyller, T.B. Prediction of long-term functional outcome after stroke rehabilitation. Clin. Rehabil. 2002, 16, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Jerrgensen, H.S.; Nakayama, H.; Reith, J.; Raaschou, H.O.; Olsen, T.S. Stroke recurrence: Predictors, severity, and prognosis. The Copenhagen Stroke Study. Neurology 1997, 48, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Öneş, K.; Yalçinkaya, E.Y.; Toklu, B.Ç.; Cağlar, N. Effects of age, gender, and cognitive, functional and motor status on functional outcomes of stroke rehabilitation. NeuroRehabilitation 2009, 25, 241–249. [Google Scholar] [CrossRef]
- Lee, K.B.; Kim, J.S.; Hong, B.Y.; Lim, S.H. Clinical recovery form stroke lesions and related outcomes. J. Clin. Neurosci. 2017, 37, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.-H.; Chua, K.S.; Tow, A.P. Clinical characteristics and functional outcome of stroke patients 75 years old and older. Arch. Phys. Med. Rehabil. 1998, 79, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Heruti, R.; Lusky, A.; Dankner, R.; Ring, H.; Dolgopiat, M.; Barell, V.; Levenkrohn, S.; Adunsky, A. Rehabilitation outcome of elderly patients after a first stroke: Effect of cognitive status at admission on the functional outcome. Arch. Phys. Med. Rehabil. 2002, 83, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Pollard, B.; Morrison, V.; MacWalter, R. Functional limitations and survival following stroke: Psychological and clinical predictors of 3-year outcome. Int. J. Behav. Med. 2004, 11, 187–196. [Google Scholar] [CrossRef]
- Knoflach, M.; Matosevic, B.; Rucker, M.; Furtner, M.; Mair, A.; Wille, G.; Zangerle, A.; Werner, P.; Ferrari, J.; Schmidauer, C.; et al. Functional recovery after ischemic stroke—A matter of age: Data from the Austrian Stroke Unit Registry. Neurology 2012, 78, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.-H.; Lee, J. Temporal recovery of activities of daily living in the first year after ischemic stroke: A prospective study of patients admitted to a rehabilitation unit. Neurorehabilitation 2014, 35, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Shen, F.; Gabriel, R.A.; Law, D.; Yang, E.Y.; Yang, G.-Y.; Young, W.L.; Su, H. Attenuation of brain response to vascular endothelial growth factor-mediated angiogenesis and neurogenesis in aged mice. Stroke 2009, 40, 3596–3600. [Google Scholar] [CrossRef]
- Hermann, D.M.; Chopp, M. Promoting brain remodelling and plasticity for stroke recovery: Therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012, 11, 369–380. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Jung, H.Y.; Park, B.K.; Shin, H.S.; Kang, Y.K.; Pyun, S.B.; Paik, N.J.; Kim, S.H.; Kim, T.H.; Han, T.R. Development of the Korean version of Modified Barthel Index (K-MBI): Multi-center study for subjects with stroke. J. Korean Acad. Rehabil. Med. 2007, 31, 283–297. [Google Scholar]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R. Gait Assessment for Neurologically Impaired Patients. Phys. Ther. 1986, 66, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.; Godwin, J.; Richards, S.; Warlow, C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: Final results. J. Neurol. Neurosurg. Psychiatry 1991, 54, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Medical Research Council. Aids to Examination of the Peripheral Nervous System; Her Majesty’s Stationary Office: London, UK, 1976. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Maaijwee, N.A.M.M.; Rutten-Jacobs, L.C.; Schaapsmeerders, P.; Van Dijk, E.J.; De Leeuw, F.-E. Ischaemic stroke in young adults: Risk factors and long-term consequences. Nat. Rev. Neurol. 2014, 10, 315–325. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Maaravi, Y.; Cohen, A.; Bursztyn, M.; Ein-Mor, E.; Stessman, J. Changing Profile of Health and Function from Age 70 to 85 Years. Gerontology 2012, 58, 313–321. [Google Scholar] [CrossRef]
- Forman, D.E.; Berman, A.D.; McCabe, C.H.; Baim, D.S.; Wei, J.Y. PTCA in the Elderly: The “Young-Old” versus the “Old-Old”. J. Am. Geriatr. Soc. 1992, 40, 19–22. [Google Scholar] [CrossRef]
- Duncan, P.W.; Goldstein, L.B.; Matchar, D.B.; Divine, G.W.; Feussner, J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 1992, 23, 1084–1089. [Google Scholar] [CrossRef]
- Lee, K.-B.; Lim, S.H.; Kim, K.H.; Kim, K.J.; Kim, Y.R.; Chang, W.N.; Yeom, J.W.; Kim, Y.D.; Hwang, B.Y. Six-month functional recovery of stroke patients. Int. J. Rehabil. Res. 2015, 38, 173–180. [Google Scholar] [CrossRef]
- Sim, T.; Lum, C.; Sze, F.K.; Or, K.; Woo, J. Outcome after stroke rehabilitation in Hong Kong. Clin. Rehabil. 1997, 11, 236–242. [Google Scholar] [CrossRef]
| Total (n = 111) | |
|---|---|
| Age, years | 58.2 ± 11.5 |
| Female sex | 52 (46.8) |
| Stroke type | |
| Ischemic | 61 (55.0) |
| Hemorrhagic | 50 (45.0) |
| Lesion side, left side | 65 (58.6) |
| Lesion location a | |
| Cortical | 51 (45.9) |
| Subcortical | 62 (55.9) |
| Brainstem | 10 (9.0) |
| Cerebellum | 9 (8.1) |
| Coexisting conditions a | |
| Atrial fibrillation | 10 (9.0) |
| Hypertension | 54 (48.6) |
| Diabetes mellitus | 22 (19.8) |
| Dyslipidemia | 32 (29.4) |
| Smoking history | 31 (27.9) |
| Alcohol history | 46 (41.4) |
| Age | Time Course | ||||
|---|---|---|---|---|---|
| 2 Weeks | 1 Month | 6 Months | 30 Months | ||
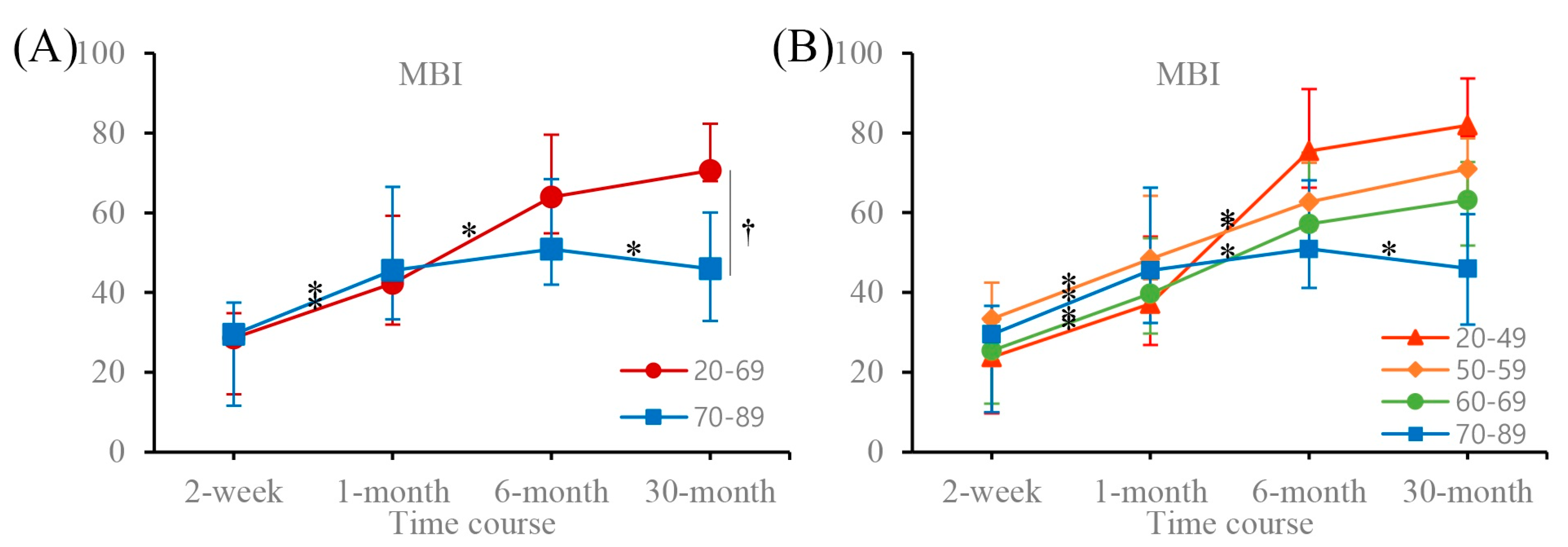
| MBI | 20–69 | 28.59 (20.31–32.24) | 42.33 * (38.77–52.75) | 63.98 * (60.99–73.29) | 70.58 (65.54–76.62) |
| 70–89 | 29.50 (11.61–37.44) | 45.57 * (33.32–66.51) | 50.91 (41.97–68.40) | 46.00 *,† (32.84–60.03) | |
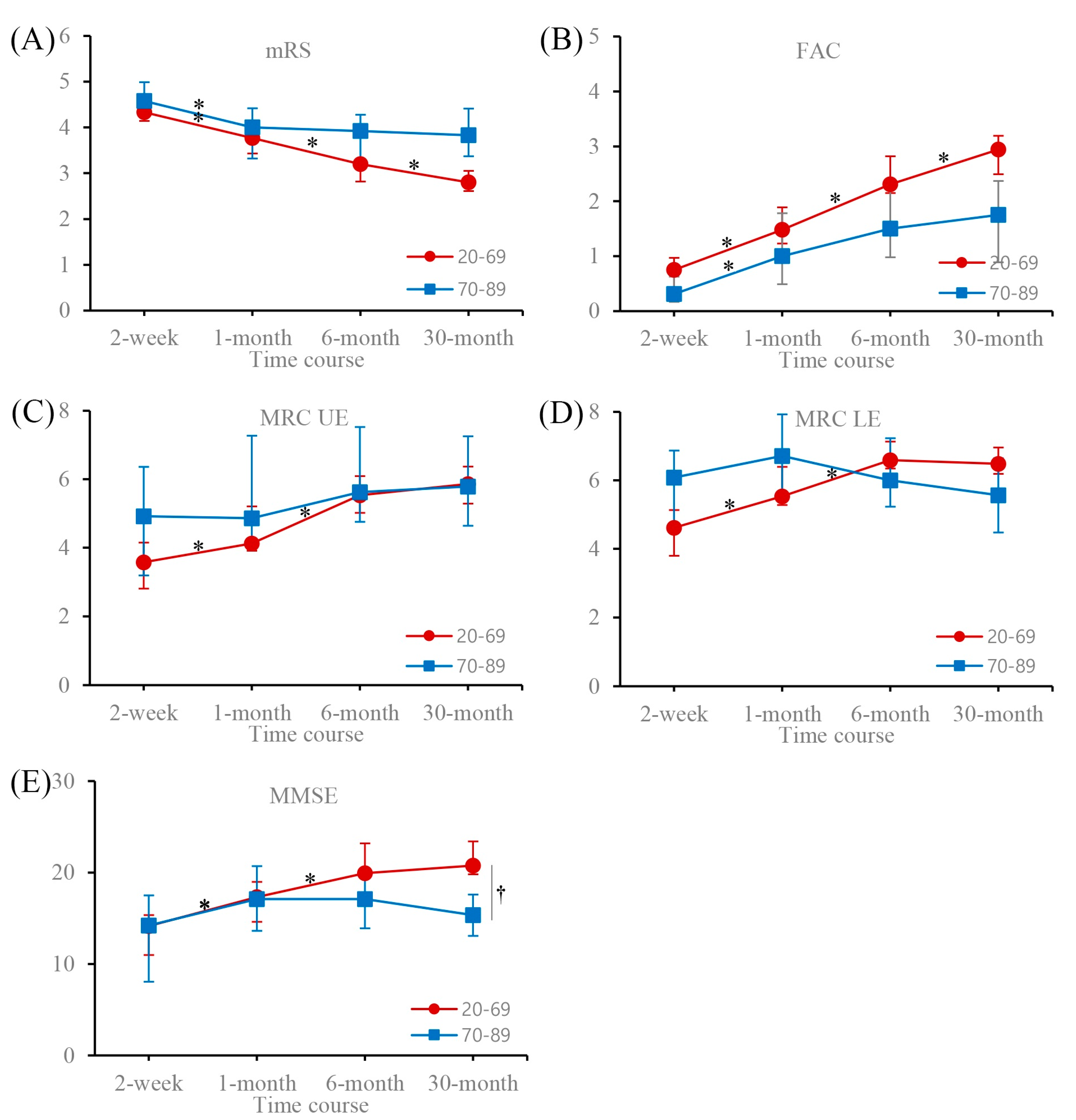
| mRS | 20–69 | 4.33 (4.14–4.59) | 3.77 * (3.43–3.92) | 3.20 * (2.82–3.28) | 2.80 * (2.61–3.05) |
| 70–89 | 4.58 (4.30–4.99) | 4.00 * (3.32–4.42) | 3.92 (3.31–4.28) | 3.83 † (3.37–4.41) | |
| FAC | 20–69 | 0.75 (0.38–0.97) | 1.48 * (1.23–1.89) | 2.31 * (2.15–2.82) | 2.94 * (2.49–3.19) |
| 70–89 | 0.31 (0.17–0.63) | 1.00 * (0.49–1.78) | 1.50 (0.98–2.33) | 1.75 (0.89–2.37) | |
| MRCUF | 20–69 | 3.58 (2.81–4.15) | 4.13 * (3.91–5.21) | 5.53 * (5.02–6.09) | 5.86 (5.29–6.37) |
| 70–89 | 4.92 (3.19–6.36) | 4.86 (4.27–7.27) | 5.62 (4.76–7.52) | 5.79 (4.64–7.25) | |
| MRCLE | 20–69 | 4.62 (3.80–5.13) | 5.53 * (5.28–6.39) | 6.59 * (6.34–7.13) | 6.48 (6.19–6.96) |
| 70–89 | 6.08 (4.72–6.87) | 6.71 (5.53–7.92) | 6.00 (5.23–7.23) | 5.57 (4.48–6.65) | |
| MMSE | 20–69 | 14.15 (10.98–15.34) | 17.33 * (14.62–18.98) | 19.93 * (19.40–23.20) | 20.76 (19.81–23.40) |
| 70–89 | 14.22 (8.06–17.49) | 17.11 * (13.64–20.71) | 17.10 (13.92–20.12) | 15.36 † (13.07–17.61) | |
| Age | Time Course | ||||
|---|---|---|---|---|---|
| 2 Weeks | 1 Month | 6 Months | 30 Months | ||
| MBI | 20–49 | 23.75 (9.65–30.00) | 37.17 * (26.83–54.04) | 75.45 * (66.28–91.03) | 81.91 (79.26–93.61) |
| 50–59 | 33.37 (22.8–42.42) | 48.37 * (43.30–64.25) | 62.65 * (55.63–72.51) | 70.92 (62.39–78.65) | |
| 60–69 | 25.36 (12.12–32.67) | 39.70 * (29.66–53.56) | 57.14 * (50.57–75.06) | 63.17 (51.76–72.70) | |
| 70–89 | 29.50 (9.97–36.68) | 45.57 * (32.32–66.27) | 50.91 (41.14–68.15) | 46.00 * (31.96–59.60) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.W.; Hong, B.Y.; Jo, L.; Kim, J.-S.; Park, J.G.; Shin, B.K.; Lim, S.H. Effects of Age on Long-Term Functional Recovery in Patients with Stroke. Medicina 2020, 56, 451. https://doi.org/10.3390/medicina56090451
Yoo JW, Hong BY, Jo L, Kim J-S, Park JG, Shin BK, Lim SH. Effects of Age on Long-Term Functional Recovery in Patients with Stroke. Medicina. 2020; 56(9):451. https://doi.org/10.3390/medicina56090451
Chicago/Turabian StyleYoo, Jae Wan, Bo Young Hong, Leechan Jo, Joon-Sung Kim, Jung Geun Park, Bo Kyung Shin, and Seong Hoon Lim. 2020. "Effects of Age on Long-Term Functional Recovery in Patients with Stroke" Medicina 56, no. 9: 451. https://doi.org/10.3390/medicina56090451
APA StyleYoo, J. W., Hong, B. Y., Jo, L., Kim, J.-S., Park, J. G., Shin, B. K., & Lim, S. H. (2020). Effects of Age on Long-Term Functional Recovery in Patients with Stroke. Medicina, 56(9), 451. https://doi.org/10.3390/medicina56090451






