Long-Term Prognostic Significance of High-Sensitive Troponin I Increase during Hospital Stay in Patients with Acute Myocardial Infarction and Non-Obstructive Coronary Arteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Markers of Myocardial Injury
2.3. Working Diagnosis, Etiology, and Discharge Diagnosis
2.4. Echocardiography
2.5. Coronary Angiography
2.6. Study Endpoints
2.7. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Clinical Characteristics
3.3. Biochemical Parameters
3.4. Type of Acute Coronary Syndrome, Electrocardiography, and Echocardiography
3.5. Coronary Artery Angiography
3.6. Working Etiology and Discharge Diagnosis
3.7. Clinical Outcomes
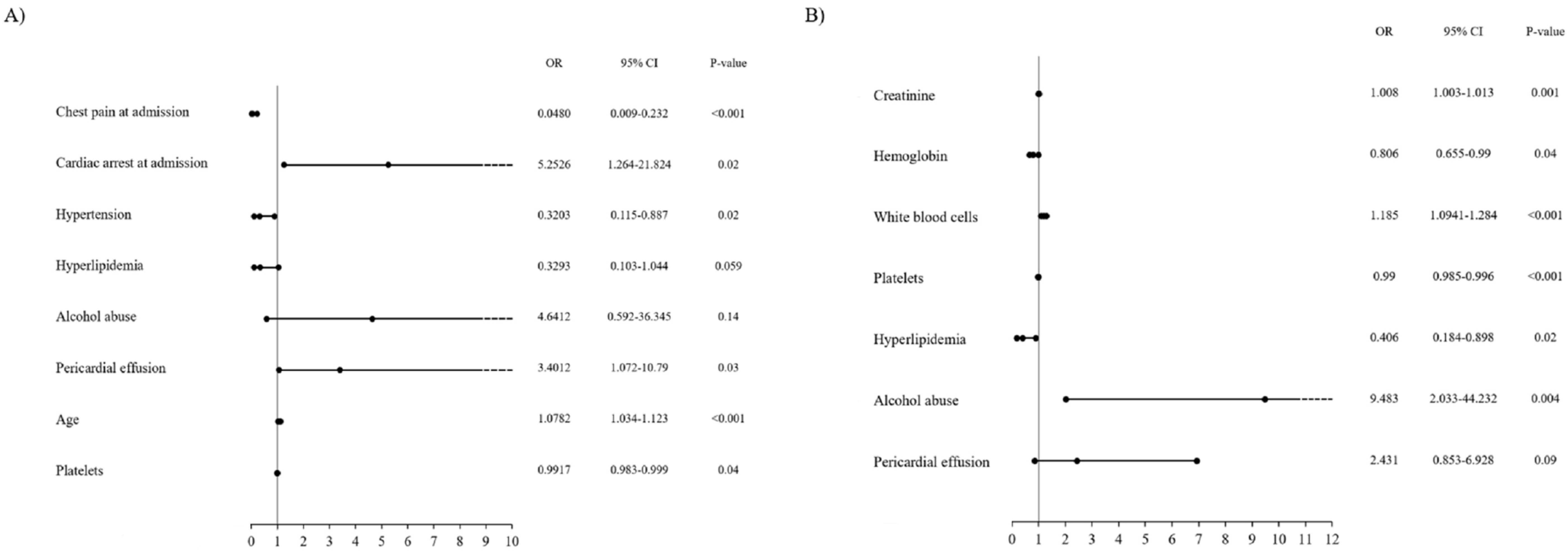
3.8. Predictors of Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adamson, D.P.; McAllister, D.; Pilbrow, A.; Pickering, W.J.; Poppe, K.; Shah, A.; Whalley, G.; Ellis, C.; Mills, L.N.; Newby, E.D.; et al. Convalescent troponin and cardiovascular death following acute coronary syndrome. Heart 2019, 105, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.R.; Fujisawa, T.; Lee, K.K.; Andrews, J.P.; Anand, A.; Sandeman, D.; Ferry, A.V.; Stewart, S.; Marshall, L.; Strachan, F.E.; et al. Novel high-sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart 2019, 105, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.R.; Lee, K.K.; McAllister, D.A.; Cullen, L.; Greenslade, J.H.; Parsonage, W.; Worster, A.; Kavsak, P.A.; Blankenberg, S.; Neumann, J.; et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA 2017, 318, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.R.; Hesse, K.; Andrews, J.; Ken Lee, K.; Anand, A.; Shah, A.S.; Sandeman, D.; Ferry, A.V.; Jameson, J.; Piya, S.; et al. High-Sensitivity cardiac troponin I and clinical risk scores in patients with suspected acute coronary syndrome. Circulation 2018, 138, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.; Lindahl, B.; Baron, T.; Jernberg, T.; Tornvall, P.; Eggers, K.M. Prognosis in relation to high-sensitivity cardiac troponin T levels in patients with myocardial infarction and non-obstructive coronary arteries. Am. Heart J. 2018, 200, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjöld, A.M.; Baron, T.; Eggers, K.M.; Jernberg, T.; Lindahl, B. Predictors of adverse outcome in patients with myocardial infarction with non-obstructive coronary artery (MINOCA) disease. Int. J. Cardiol. 2018, 261, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. WG on cardiovascular pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; Van de Werf, F.; et al. ESC scientific document group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, K. Angina pectoris-myocardial infarction investigations in Japan. Transient left ventricular apical ballooning without coronary arterystenosis: A novel heart syndrome mimicking acute myocardial infarction. Angina pectoris-myocardial infarction investigations in Japan. J. Am. Coll. Cardiol. 2001, 38, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Samman Tahhan, A.; Sandesara, P.; Hayek, S.S.; Hammadah, M.; Alkhoder, A.; Kelli, H.M.; Topel, M.; O’Neal, W.T.; Ghasemzadeh, N.; Ko, Y.A.; et al. High sensitivity troponin I Levels and coronary artery disease severity, progression, and long-term outcomes. J. Am. Heart Assoc. 2018, 7, e007914. [Google Scholar] [CrossRef] [PubMed]
- Kaura, A.; Panoulas, V.; Glampson, B.; Davies, J.; Mulla, A.; Woods, K.; Omigie, J.; Shah, A.D.; Channon, K.M.; Weber, J.N.; et al. Association of troponin level and age with mortality in 250,000 patients: Cohort study across five UK acute care centres. BMJ 2019, 367, l6055. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Venge, P.; Wallentin, L. Relation between troponin T and the risk of subsequent cardiac events in unstable coronary artery disease. The FRISC study group. Circulation 1996, 93, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.G.; Kirchberger, I.; Amann, U.; Heier, M.; Thilo, C.; Kuch, B.; Peters, A.; Meisinger, C. Association between admission anemia and long-term mortality in patients with acute myocardial infarction: Results from the MONICA/KORA myocardial infarction registry. BMC Cardiovasc. Disord. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Wańha, W.; Kawecki, D.; Roleder, T.; Pluta, A.; Marcinkiewicz, K.; Dola, J.; Morawiec, B.; Krzych, Ł.; Pawłowski, T.; Smolka, G.; et al. Impact of anaemia on long-term outcomes in patients treated with first-and second-generation drug-eluting stents; Katowice-Zabrze Registry. Kardiol. Pol. 2016, 74, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Sinkovič, A.; Majal, M. The impact of thrombocytopenia on outcome in patients with acute coronary syndromes: A single center retrospective study. BioMed Res. Int. 2015, 2015, 907304. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.; Kaplan, S.T.; Kiris, A.; Gedikli, O. Impact of initial platelet count on baseline angiographic finding and end-points in ST-elevation myocardial infarction referred for primary percutaneous coronary intervention. Int. J. Clin. Exp. Med. 2014, 7, 1064–1070. [Google Scholar] [PubMed]
- Mueller, C.; Neumann, F.J.; Hochholzer, W.; Trenk, D.; Zeller, T.; Perruchoud, A.P.; Buettner, H.J. The impact of platelet count on mortality in unstable angina/non-ST-segment elevation myocardial infarction. Am. Heart J. 2006, 151, 1214-e1. [Google Scholar] [CrossRef] [PubMed]
- Milwidsky, A.; Ziv-Baran, T.; Letourneau-Shesaf, S.; Keren, G.; Taieb, P.; Berliner, S.; Shacham, Y. CRP velocity and short-term mortality in ST segment elevation myocardial infarction. Biomarkers 2017, 22, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Pesaro, A.E.P.; Nicolau, J.C.; Serrano, C.V., Jr.; Truffa, R.; Gaz, M.V.B.; Karbstein, R.; Giraldez, R.R.; Kalil Filho, R.; Ramires, J.A. Influence of leukocytes and glycemia on the prognosis of patients with acute myocardial infarction. Arq. Bras. Cardiol. 2009, 92, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Hiasa, Y.; Ohara, Y.; Miyazaki, S.I.; Ogura, R.; Suzuki, N.; Hosokawa, S.; Kishi, K.; Ohtani, R. Relationship of admission neutrophil count to microvascular injury, left ventricular dilation, and long-term outcome in patients treated with primary angioplasty for acute myocardial infarction. Circ. J. 2008, 72, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Faridi, K.F.; Joshi, P.H.; Blaha, M.J.; Kulkarni, K.R.; Khokhar, A.A.; Maddox, T.M.; Havranek, E.P.; Toth, P.P.; Tang, F.; et al. Remnant lipoprotein cholesterol and mortality after acute myocardial infarction: Further evidence for a hypercholesterolemia paradox from the TRIUMPH registry. Clin. Cardiol. 2015, 38, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Velavan, P.; Huan Loh, P.; Clark, A.; Cleland, J.G. The cholesterol paradox in heart failure. Congest. Heart Fail. 2007, 13, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Jędrychowska, M.; Januszek, R.; Plens, K.; Surdacki, A.; Bartuś, S.; Dudek, D. Impact of sex on the follow-up course and predictors of clinical outcomes in patients hospitalised due to myocardial infarction with non-obstructive coronary arteries: A single-centre experience. Kardiol. Pol. 2019, 77, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Albani, S.; Fabris, E.; Stolfo, D.; Falco, L.; Barbati, G.; Aquaro, G.D.; Vitrella, G.; Rakar, S.; Korcova, R.; Lardieri, G.; et al. Prognostic relevance of pericardial effusion in STEMI patients treated by primary percutaneous coronary intervention: A 10-year single-centre experience. Eur. Heart J. Acute Cardiovasc. Care 2019, 2048872619884858. [Google Scholar] [CrossRef] [PubMed]
- Jobs, A.; Eitel, C.; Pöss, J.; Desch, S.; Thiele, H.; Eitel, I. Effect of pericardial effusion complicating st-elevation myocardial infarction as predictor of extensive myocardial damage and prognosis. Am. J. Cardiol. 2015, 116, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Pai, J.K.; Mukamal, K.J.; Rimm, E.B. Long-Term alcohol consumption in relation to all-cause and cardiovascular mortality among survivors of myocardial infarction: The health professionals follow-up study. Eur. Heart J. 2012, 33, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B. Alcoholic cardiomyopathy: The result of dosage and individual predisposition. Herz 2016, 41, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Homorodean, C.; Iancu, A.C.; Dregoesc, I.M.; Spînu, M.; Ober, M.C.; Tãtaru, D.; Leucuţa, D.; Olinic, M.; Olinic, D.M. Renal failure impact on the outcomes of ST-segment elevation myocardial infarction patients due to a left main coronary culprit lesion treated using a primary percutaneous coronary intervention. J. Clin. Med. 2019, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Plakht, Y.; Shiyovich, A.; Gilutz, H. Predictors of long-term (10-year) mortality post myocardial infarction: Age-related differences. Soroka Acute Myocardial Infarction (SAMI) Project. J. Cardiol. 2015, 65, 216–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sullivan, A.K.; Holdright, D.R.; Wright, C.A.; Sparrow, J.L.; Cunningham, D.; Fox, K.M. Chest pain in women: Clinical, investigative, and prognostic features. BMJ 1994, 308, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D.; Shaw, L.J.; Pepine, C.J.; Reis, S.E.; Kelsey, S.F.; Sopko, G.; Rogers, W.J.; Mankad, S.; Sharaf, B.L.; Bittner, V.; et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: Results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur. Heart J. 2006, 27, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Khuddus, M.A.; Pepine, C.J.; Handberg, E.M.; Baireymerz, C.N.; Sopko, G.; Bavry, A.A.; Denardo, S.J.; McGorray, S.P.; Smith, K.M.; Sharaf, B.L.; et al. An intravascular ultrasound analysis in women experiencing chestpain in the absence of obstructive coronary artery disease: A substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J. Interv. Cardiol. 2010, 23, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mygind, N.D.; Michelsen, M.M.; Pena, A.; Frestad, D.; Dose, N.; Aziz, A.; Faber, R.; Høst, N.; Gustafsson, I.; Hansen, P.R.; et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: The iPOWER study. J. Am. Heart Assoc. 2016, 5, e003064. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Concentration of High-Sensitivity Cardiac I Troponin (Increase—Times above the Upper Normal Limit) | p-Value | ||
|---|---|---|---|---|
| ≤5 | >5 to ≤20 | >20 | ||
| Age, years | 69.1 ± 10.6 | 63.9 ± 12.9 | 62.6 ± 16.4 | 0.008 |
| Hospitalization duration, days | 4.58 ± 2.38 | 5.38 ± 3.45 | 6.06 ± 3.42 | 0.02 |
| Gender, female | 41 (47.1) | 39 (50.6) | 79 (45.7) | 0.76 |
| Arterial hypertension | 72 (83.7) | 64 (83,1) | 116 (67.8) | 0.004 |
| Hyperlipidemia | 57 (66.3) | 35 (45.4) | 61 (35.9) | <0.001 |
| Diabetes | 30 (34.9) | 16 (20.8) | 38 (22.2) | 0.052 |
| Kidney failure | 15 (17.6) | 7 (9.1) | 19 (11.2) | 0.2 |
| Atrial fibrillation | 17 (19.5) | 15 (19.5) | 25 (14.5) | 0.46 |
| COPD/Asthma | 9 (10.5) | 8 (10.4) | 14 (8.2) | 0.77 |
| Autoimmune disease | 2 (2,3) | 5 (6.5) | 7 (4.1) | 0.45 |
| Oncological disease | 8 (9.3) | 7 (9.1) | 11 (6.5) | 0.65 |
| Hypercoagulable state | 6 (7) | 3 (3.9) | 3 (1.8) | 0.13 |
| Smoking | 22 (26.5) | 19 (35.2) | 12 (29.3) | 0.55 |
| Alcohol abuse | 2 (2.3) | 2 (2.6) | 7 (4.3) | 0.67 |
| Prior myocardial infarction | 30 (34.5) | 16 (20.8) | 37 (21.5) | 0.04 |
| Prior PCI | 32 (36.8) | 12 (15.6) | 27 (15.8) | <0.001 |
| Cardiac arrest | 3 (3.4) | 0 (0) | 15 (8.8) | 0.01 |
| Prior cerebral stroke/TIA | 6 (6.9) | 4 (5.2) | 12 (7) | 0.86 |
| Prior DVT/PE | 2 (2.3) | 0 (0) | 4 (2.3) | 0.4 |
| Pharmacotherapy | ||||
| Acetyl salicylic acid | 33 (55) | 26 (42.6) | 33 (22.9) | 0.04 |
| P2Y12 blockers | 14 (25) | 8 (13.6) | 10 (7) | 0.002 |
| Anticoagulants | 17 (28.3) | 15 (25) | 14 (9.9) | 0.001 |
| Beta-blocker | 38 (59.4) | 28 (43.7) | 42 (29.2) | <0.001 |
| Statins | 38 (59.4) | 27 (44.3) | 36 (25) | <0.001 |
| Biochemical Analyses | ||||
| White blood cells, 103/µL | 8.18 ± 2.98 | 8.8 ± 3.12 | 9.98 ± 4.8 | 0.02 |
| Platelet count,·103/µL | 225.9 ± 98 | 239.8 ± 79.8 | 220.5 ± 79.8 | 0.12 |
| Hemoglobin, g/dL | 13.5 ± 1.6 | 13.1 ± 1.8 | 12.9 ± 2.1 | 0.15 |
| C-reactive protein, mg/L | 13.3 ± 9.8 | 44.4 ± 53.9 | 41.1 ± 59.1 | 0.16 |
| Total Cholesterol, mmol/L | 4.15 ± 1.02 | 4.75 ± 1.1 | 4.5 ± 1.1 | 0.01 |
| Cholesterol HDL, mmol/L | 1.25 ± 0.4 | 1.34 ± 0.4 | 1.24 ± 0.4 | 0.45 |
| Cholesterol LDL, mmol/L | 2.24 ± 0.8 | 2.66 ± 1 | 2.58 ± 1.1 | 0.4 |
| Triglycerides, mmol/L | 1.48 ± 0.8 | 1.68 ± 1.1 | 1.49 ± 0.8 | 0.79 |
| eGFR < 60 mL/min. | 20 (25.6) | 13 (19.1) | 34 (25.9) | 0.53 |
| Creatinine, µmol/L | 76.5 ± 34.6 | 68.9 ± 14.5 | 83.56 ± 43.9 | 0.23 |
| CK-MB at admission, U/I | 1.5 ± 3.1 | 11.7 ± 15.2 | 44 ± 75 | <0.001 |
| CK-MB max., U/I | 30.4 ± 23.1 | 28.1 ± 16.4 | 68.6 ± 160 | <0.001 |
| Concentration of High-Sensitivity Cardiac I Troponin (Increase—Times above the Upper Normal Limit) | p-Value | |||
|---|---|---|---|---|
| ≤5 | >5 to ≤20 | >20 | ||
| Type of Myocardial Infarction at Admission—Working Diagnosis | ||||
| STEMI | 8 (9.3) | 9 (11.5) | 37 (21.4) | <0.001 |
| NSTEMI | 69 (80.2) | 68 (87.2) | 136 (78.6) | |
| Unstable angina | 9 (10.5) | 0 | 0 | |
| Electrocardiography | ||||
| ST segment elevation/LBBB | 13 (15.3) | 18 (23.4) | 44 (25.6) | 0.17 |
| ST segment depression | 21 (24.7) | 20 (26) | 29 (16.9) | 0.16 |
| T-wave inversion | 15 (20.3) | 14 (18.7) | 34 (20.1) | 0.95 |
| Echocardiography | ||||
| LVEF ≥ 40% | 49 (59.8) | 60 (80) | 114 (66,67) | 0.02 |
| Mean LVEF | 43.6 ±15.8 | 52.1 ±11.8 | 47.8 ±14.3 | 0.001 |
| Normal contractility | 33 (39.8) | 30 (40) | 41 (24.4) | 0.01 |
| Present hypokinesis | 32 (38.5) | 26 (35.1) | 72 (42.9) | 0.05 |
| Present akinesis | 18 (21.7) | 18 (24.3) | 55 (32.7) | 0.13 |
| Pericardial effusion | 2 (2.4) | 5 (6.8) | 15 (8.9) | 0.15 |
| Coronary Angiography | ||||
| Vascular access, radial | 65 (75.6) | 54 (76.1) | 115 (78.8) | 0.82 |
| Non-stenotic plaques | 65 (74.6) | 59 (76.6) | 119 (75.8) | 0.95 |
| Slow-flow contrasts | 2 (2.3) | 4 (5.2) | 19 (11) | 0.02 |
| Eccentric plaque | 2 (2.3) | 3 (3.9) | 1 (0.6) | 0.17 |
| Myocardial bridges | 4 (4.6) | 4 (5.2) | 10 (5.8) | 0.92 |
| Arterial spasm | 1 (1.1) | 0 (0) | 5 (2.9) | 0.42 |
| Thrombus | 0 (0) | 0 (0) | 2 (1.1) | 0.38 |
| Etiology | Concentration of High-Sensitivity Cardiac I Troponin (Increase—Times above the Upper Normal Limit) | p-Value | ||
|---|---|---|---|---|
| ≤5 | >5 to ≤20 | >20 | ||
| Unknown | 14 (16.1) | 18 (23.4) | 58 (33.4) | 0.008 |
| Arterial spasm | 0 (0) | 1 (1.3) | 4 (2.3) | 0.34 |
| Myocarditis | 1 (1.2) | 5 (6.5) | 14 (8.1) | 0.07 |
| HCM | 2 (2.3) | 1 (1.3) | 2 (1.2) | 0.76 |
| Takotsubo cardiomyopathy | 0 (0) | 4 (5.2) | 27 (15.5) | <0.001 |
| Slow-flow phenomenon | 0 (0) | 2 (2.6) | 8 (4.6) | 0.11 |
| AV conduction disorders | 3 (3.4) | 3 (3.9) | 2 (1.2) | 0.31 |
| Aortic dissection | 0 (0) | 1 (1.3) | 1 (0.6) | 0.55 |
| Tachyarrhythmias | 10 (12.6) | 6 (7.8) | 19 (11) | 0.69 |
| Atrial fibrillation | 10 (12.6) | 6 (7.8) | 15 (8.7) | 0.67 |
| Arterial hypertension | 13 (14.9) | 9 (11.7) | 4 (2.3) | <0.001 |
| Anemia | 0 (0) | 0 (0) | 2 (1.2) | 0.38 |
| Oncological embolization | 1 (1.2) | 1 (1.3) | 3 (1.7) | 0.92 |
| Myocardial bridge | 2 (2.3) | 2 (2.6) | 3 (1.7) | 0.89 |
| PE/DVT | 0 (0) | 0 (0) | 1 (0.6) | 0.62 |
| Muscular dystrophy | 0 (0) | 0 (0) | 1 (0.6) | 0.62 |
| Cerebral stroke | 0 (0) | 0 (0) | 1 (0.6) | 0.62 |
| Alcoholic cardiomyopathy | 0 (0) | 0 (0) | 1 (0.6) | 0.62 |
| Aortic valve stenosis | 2 (2.3) | 1 (1.3) | 2 (1.2) | 0.76 |
| Antiphospholipid syndrome | 0 (0) | 0 (0) | 1 (0.6) | 0.62 |
| Vasculitis | 0 (0) | 0 (0) | 1 (0.6) | 0.62 |
| Selected Indices | Concentration of High-Sensitivity Cardiac I Troponin (Increase—Times above the Upper Normal Limit) | p-Value | ||
|---|---|---|---|---|
| ≤5 | >5 to ≤20 | >20 | ||
| Diagnosis at Discharge from Hospital | ||||
| Myocarditis | 1 (1.1) | 4 (5.2) | 13 (7.5) | 0.09 |
| Takotsubo cardiomyopathy | 0 (0) | 1 (1.3) | 21 (12.1) | <0.001 |
| Arrhythmias | 5 (5.8) | 5 (6.5) | 4 (2.3) | 0.21 |
| Atrial fibrillation | 5 (5.8) | 5 (6.5) | 3 (1.7) | 0.11 |
| Arterial hypertension | 7 (8.1) | 5 (6.5) | 2 (1.2) | 0.01 |
| Venous thromboembolic disease | 0 (0) | 0 (0) | 1 (0.6) | 0.62 |
| NSTEMI | 38 (43.7) | 47 (61) | 83 (48) | 0.06 |
| STEMI | 3 (3.4) | 4 (5.2) | 14 (8.1) | 0.31 |
| Heart failure | 27 (31) | 3 (3.9) | 20 (11.5) | <0.001 |
| Type 2 myocardial infarction | 5 (5.8) | 6 (7.8) | 12 (6.9) | 0.87 |
| Hypertrophic cardiomyopathy | 1 (1.1) | 1 (1.3) | 1 (0.6) | 0.81 |
| Myocardial bridge | 0 (0) | 1 (1.3) | 1 (0.6) | 0.55 |
| Cerebral stroke | 0 (0) | 0 (0) | 1 (0.6) | 0.62 |
| Follow-Up | ||||
| Mean time of follow-up, days | 482 ± 173 | 461 ± 245 | 566 ± 382 | 0.23 |
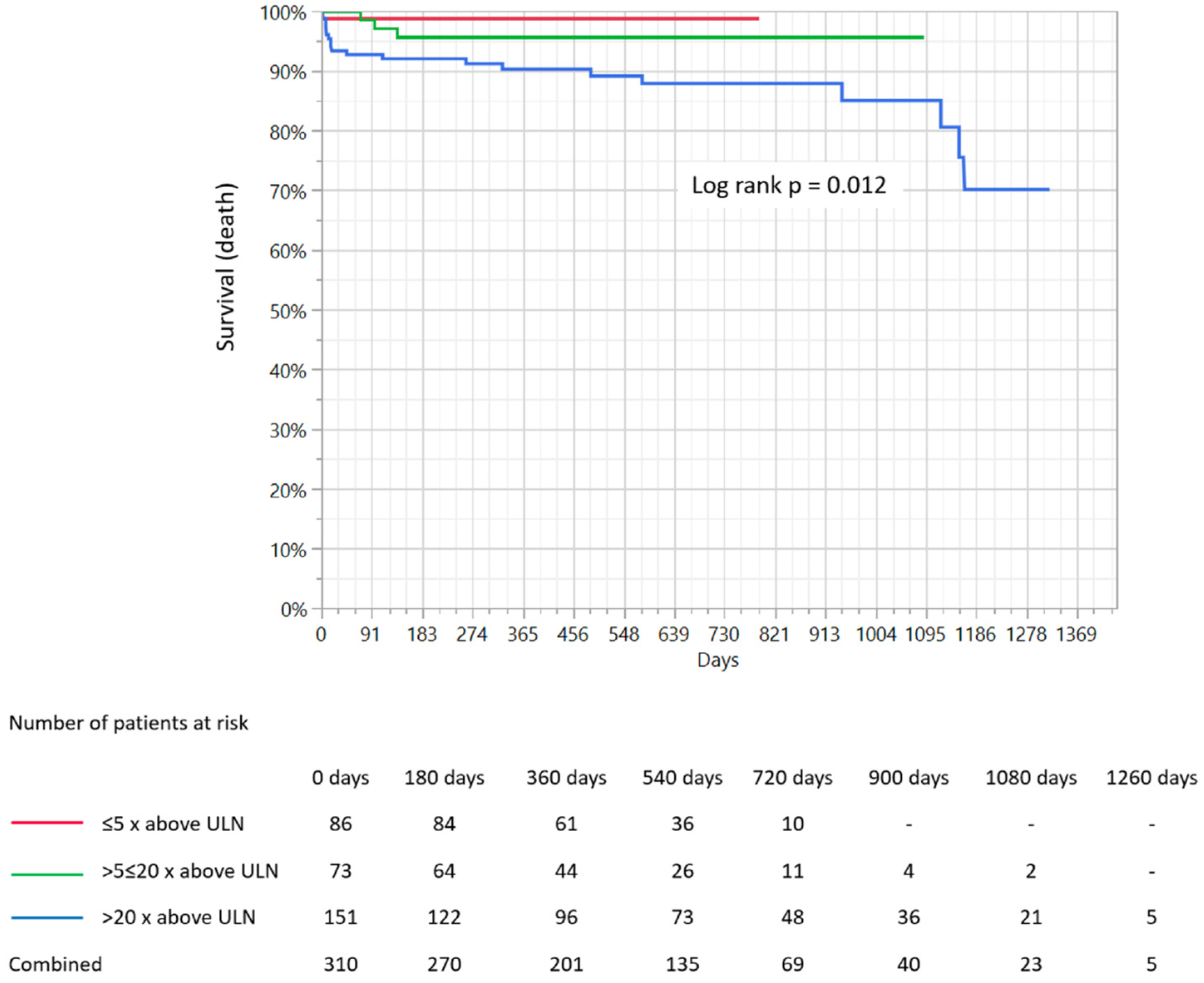
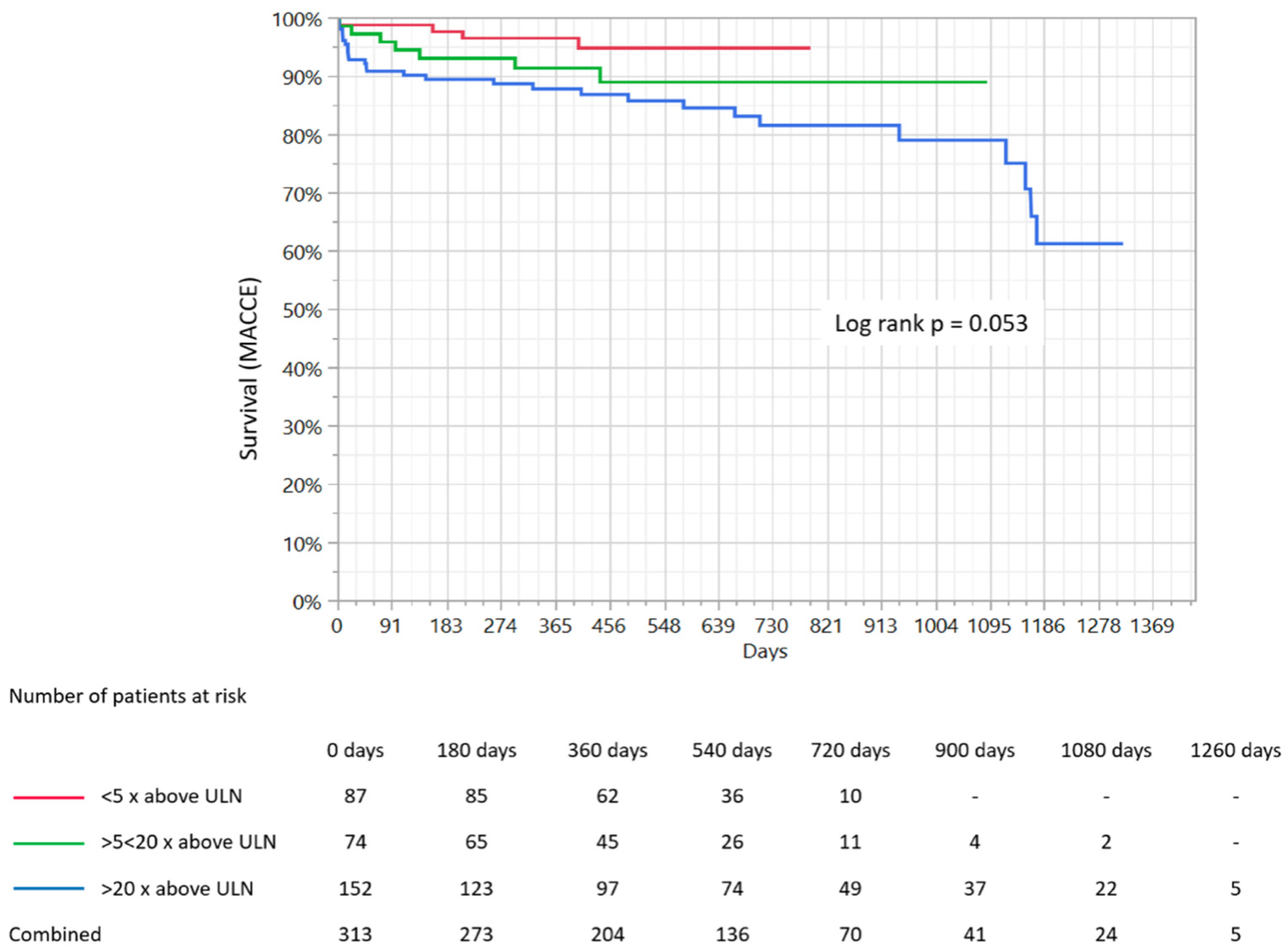
| MACCE | 4 (4.6) | 7 (9.4) | 28 (18.4) | 0.005 |
| Overall all-cause mortality | 1 (1.1) | 3 (4) | 20 (13.1) | 0.001 |
| In-hospital all-cause mortality | 1 (1.1) | 1 (1.3) | 6 (3.5) | 0.39 |
| Myocardial infarction | 4 (4.6) | 3 (4) | 24 (15.8) | 0.003 |
| PCI | 0 (0) | 0 (0) | 4 (2.6) | 0.11 |
| Cerebral stroke/TIA | 0 (0) | 3 (4) | 0 (0) | 0.007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrychowska, M.; Januszek, R.; Wańha, W.; Malinowski, K.P.; Kunik, P.; Trznadel, A.; Bartuś, J.; Staszczak, B.; Januszek, S.M.; Kameczura, T.; et al. Long-Term Prognostic Significance of High-Sensitive Troponin I Increase during Hospital Stay in Patients with Acute Myocardial Infarction and Non-Obstructive Coronary Arteries. Medicina 2020, 56, 432. https://doi.org/10.3390/medicina56090432
Jędrychowska M, Januszek R, Wańha W, Malinowski KP, Kunik P, Trznadel A, Bartuś J, Staszczak B, Januszek SM, Kameczura T, et al. Long-Term Prognostic Significance of High-Sensitive Troponin I Increase during Hospital Stay in Patients with Acute Myocardial Infarction and Non-Obstructive Coronary Arteries. Medicina. 2020; 56(9):432. https://doi.org/10.3390/medicina56090432
Chicago/Turabian StyleJędrychowska, Magdalena, Rafał Januszek, Wojciech Wańha, Krzysztof Piotr Malinowski, Piotr Kunik, Agata Trznadel, Joanna Bartuś, Bartłomiej Staszczak, Sławomir Mateusz Januszek, Tomasz Kameczura, and et al. 2020. "Long-Term Prognostic Significance of High-Sensitive Troponin I Increase during Hospital Stay in Patients with Acute Myocardial Infarction and Non-Obstructive Coronary Arteries" Medicina 56, no. 9: 432. https://doi.org/10.3390/medicina56090432
APA StyleJędrychowska, M., Januszek, R., Wańha, W., Malinowski, K. P., Kunik, P., Trznadel, A., Bartuś, J., Staszczak, B., Januszek, S. M., Kameczura, T., Wojakowski, W., Surdacki, A., & Bartuś, S. (2020). Long-Term Prognostic Significance of High-Sensitive Troponin I Increase during Hospital Stay in Patients with Acute Myocardial Infarction and Non-Obstructive Coronary Arteries. Medicina, 56(9), 432. https://doi.org/10.3390/medicina56090432






