Pulmonologists Adherence to the Chronic Obstructive Pulmonary Disease GOLD Guidelines: A Goal to Improve
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Collection
2.3. Adherence to the GOLD Guidelines
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, 2011–2030. [Google Scholar] [CrossRef] [PubMed]
- Albitar, H.A.H.; Iyer, V.N. Adherence to Global Initiative for Chronic Obstructive Lung Disease guidelines in the real world: Current understanding, barriers, and solutions. Curr. Opin. Pulm. Med. 2020, 26, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-O.; Shin, H.-J.; Kim, Y.-I.; Rhee, C.-K.; Lee, W.-Y.; Lim, S.-Y.; Ra, S.-W.; Jung, K.-S.; Yoo, K.-H.; Park, S.-J.; et al. Adherence to the GOLD guideline in COPD management of South Korea: Findings from KOCOSS Study 2011–2018. Chonnam Med. J. 2019, 55, 47–53. [Google Scholar] [CrossRef]
- Ardelean, D.L.; Lunceanu, I.; Popescu, R.; Didilescu, C.; Dinescu, S.; Olteanu, M.; Niţu, M. Evaluation of COPD patients using CAT-COPD assessment test. Pneumologia 2012, 61, 221–229. [Google Scholar]
- Cojocaru, C.; Marian, M.I.; Cojocaru, E. La perception de la fatigue chez les patients avec broncho-pneumonie chronique obstructive. Rev. Mal. Respir. 2012, 29, A58. [Google Scholar] [CrossRef]
- Sharif, R.; Cuevas, C.R.; Wang, Y.; Arora, M.; Sharma, G. Guideline adherence in management of stable chronic obstructive pulmonary disease. Respir. Med. 2013, 107, 1046–1052. [Google Scholar] [CrossRef]
- Corrado, A.; Rossi, A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir. Med. 2012, 106, 989–997. [Google Scholar] [CrossRef]
- Sen, E.; Guclu, S.Z.; Kibar, I.; Ocal, U.; Yilmaz, V.; Celik, O.; Cimen, F.; Topcu, F.; Orhun, M.; Tereci, H.; et al. Adherence to GOLD guideline treatment recommendations among pulmonologists in Turkey. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 2657–2663. [Google Scholar] [CrossRef]
- Turan, O.; Emre, J.C.; Deniz, S.; Baysak, A.; Turan, P.A.; Mirici, A. Adherence to current COPD guidelines in Turkey. Expert Opin. Pharmacother. 2016, 17, 153–158. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Huang, S.-Y.; Yang, T.-M.; Tao, C.-W.; Cheng, S.-L.; Lee, C.-H.; Kuo, P.-H.; Wu, Y.-K.; Chen, N.-H.; Hsu, W.-H.; et al. The impact of 2011 and 2017 Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) guidelines on allocation and pharmacological management of patients with COPD in Taiwan: Taiwan Obstructive Lung Disease (TOLD) study. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Small, M.; Holmgren, U. A cross-sectional survey of current treatment and symptom burden of patients with COPD consulting for routine care according to GOLD 2014 classifications. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Palmiotti, G.A.; Lacedonia, D.; Liotino, V.; Schino, P.; Satriano, F.; Di Napoli, P.L.; Sabato, E.; Mastrosimone, V.; Scoditti, A.; Carone, M.; et al. Adherence to GOLD guidelines in real-life COPD management in the Puglia region of Italy. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Aissa, S.; Knaz, A.; Maatoug, J.; Khedher, A.; Benzarti, W.; Abdelghani, A.; Garrouche, A.; Hayouni, A.; Benzarti, M.; Gargouri, I.; et al. Adherence of North-African Pulmonologists to the 2017-Global Initiative for Chronic Obstructive Lung Disease (GOLD) Pharmacological Treatment Guidelines (PTGs) of Stable Chronic Obstructive Pulmonary Disease (COPD). BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, P.V.; Sabaté, E. Adherence to long-term therapies: Evidence for action. J. Nurs. Scholarsh. 2003, 35, 207. [Google Scholar]
- Ulmeanu, R.; Mihaltan, F.; Arghir, O.; Fira-Mladinescu, O.; Teodorescu, G. Treatment Goals in COPD: The Concordance Between Patients and Physicians (Interim Results of ACORD Study). Chest 2016, 150, 873A. [Google Scholar] [CrossRef]
- Hogea, S.-P.; Tudorache, E.; Fildan, A.P.; Fira-Mladinescu, O.; Marc, M.; Oancea, C. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin. Respir. J. 2020, 14, 183–197. [Google Scholar] [CrossRef]
- Sandu, V.M.; Mihaescu, T.; Filipeanu, D.; Cernomaz, A.; Crisan-Dabija, R.A. Impact of halotherapy on COPD exacerbations. Eur. Respir. J. 2019, 54, PA2498. [Google Scholar] [CrossRef]
- Asche, C.V.; Leader, S.; Plauschinat, C.; Raparla, S.; Yan, M.; Ye, X.; Young, D. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled cortico-steroids. Int. J. Chron. Obstruct. Pulmon. Dis. 2012, 7, 201–209. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Liu, S.-L.; Chuang, C.-H.; Jheng, Y.-H. Effects of guideline-oriented pharmacotherapy in patients with newly diagnosed COPD: A prospective study. Wien Klin. Wochenschr. 2013, 125, 353–361. [Google Scholar] [CrossRef]
- Miravitlles, M.; Sicras, A.; Crespo, C.; Cuesta, M.; Brosa, M.; Galera, J.; Lahoz, R.; Lleonart, M.; Riera, M.I. Costs of chronic obstructive pulmonary disease in relation to compliance with guidelines: A study in the primary care setting. Ther. Adv. Respir. Dis. 2013, 7, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.P.; Ko, F.W.S.; Chan, H.S.; Wong, M.L.; Mok, T.Y.W.; Choo, K.L.; Hui, D.S.C. Adherence to a COPD treatment guideline among patients in Hong Kong. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Duarte-de-Araújo, A.; Teixeira, P.; Hespanhol, V.; Correia-de-Sousa, J. COPD: Analysing factors associated with a successful treatment. Pulmonology 2020, 26, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sehl, J.; O’Doherty, J.; O’Connor, R.; O’Sullivan, B.; O’Regan, A. Adherence to COPD management guidelines in general practice? A review of the literature. Ir. J. Med. Sci. 2018, 187, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Surani, S.; Aiyer, A.; Eikermann, S.; Murphy, T.; Anand, P.; Varon, J.; Vanderheiden, D.; Khan, A.; Guzman, A. Adoption and adherence to chronic obstructive pulmonary disease GOLD guidelines in a primary care setting. SAGE Open Med. 2019, 7, 1–4. [Google Scholar] [CrossRef]
- Chinai, B.; Hunter, K.; Roy, S. Outpatient Management of Chronic Obstructive Pulmonary Disease: Physician Adherence to the 2017 Global Initiative for Chronic Obstructive Lung Disease Guidelines and its Effect on Patient Outcomes. J. Clin. Med. Res. 2019, 11, 556–562. [Google Scholar] [CrossRef][Green Version]
- Ulmeanu, R.; Oancea, C.; Fildan, A.; Mihaltan, F. (Eds.) Recomandari de Diagnostic si Tratament in Bronhopneumopatia Obstructivă Cronica; Editura Medicală: Bucuresti, Romania, 2019. [Google Scholar]
- Davidescu, L.; Jurca, R.; Ulmeanu, R. Value of adding behavioural-cognitive therapy to standard treatment in smoking cessation programme: Results of Smoking Cessation Centre Oradea on 7 years. Eur. Respir. J. 2014, 44, P4161. [Google Scholar]
- U.S. National Library of Medicine. Available online: https://www.definitions.net/definition/guideline+adherence (accessed on 9 May 2020).
- López-Campos, J.L.; Gallego, E.Q.; Hernández, L.C. Status of and strategies for improving adherence to COPD treatment. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1503–1515. [Google Scholar] [CrossRef]
- Ghosh, S.; Anderson, W.H.; Putcha, N.; Han, N.K.; Curtis, J.L.; Criner, G.J.; Dransfield, M.T.; Barr, R.G.; Krishnan, J.A.; Lazarus, S.C.; et al. Alignment of COPD medication use patterns with GOLD guidelines: Analysis of the SPIROMICS cohort. In B102. Clinical Trials and Studies in COPD; American Thoracic Society: New York, NY, USA, 2018; p. A4246. [Google Scholar]
- White, P.; Thornton, H.; Pinnock, H.; Georgopoulou, S.; Booth, H.P. Overtreatment of COPD with inhaled corticosteroids-implications for safety and costs: Cross-sectional observational study. PLoS ONE 2013, 8, e75221. [Google Scholar] [CrossRef]
- Ray, R.; Hahn, B.; Stanford, R.H.; White, J.; Essoi, B.; Hunter, A.G. Classification of Patients with COPD on LAMA Monotherapy Using the GOLD Criteria: Analysis of a Claims-Linked Patient Survey Study. Pulm. Ther. 2019, 5, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Katsura, H.; Koh, E.; Hiroshima, K.; Fujisawa, T. Early detection of COPD is important for lung cancer surveillance. Eur. Respir. J. 2012, 39, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Jimborean, G.; Szasz, S.; Szathmary, M.; Csipor, A.; Arghir, O.; Nemes, R.; Postolache, P.; Ianosi, E.S. Association Between Chronic Obstructive Pulmonary Disease and Sleep Apnea—Overlap Syndrome—Experience of Pulmonology Clinic Tg. Mures, Romania. Rev. Chim. Buchar. 2018, 69, 1014–1017. [Google Scholar] [CrossRef]
- Budin, C.; Maierean, A.; Ianosi, E.S.; Socaci, A.; Buzoianu, A.D.; Alexescu, T.; Olteanu, M.; Rusu, E.; Moldovan, A.; Nemes, R.M. Nocturnal hypoxemia, a key parameter in overlap syndrome. Rev. Chim. Buchar. 2019, 70, 449–454. [Google Scholar] [CrossRef]
- Matte, D.L.; Pizzichini, M.M.M.; Hoepers, A.T.C.; Diaz, A.P.; Karloh, M.; Dias, M.; Pizzichini, E. Prevalence of depression in COPD: A systematic review and meta-analysis of controlled studies. Respir. Med. 2016, 117, 154–161. [Google Scholar] [CrossRef]
- Gershon, A.; Croxford, R.; Calzavara, A.; To, T.; Stanbrook, M.B.; Upshur, R.; Stukel, T.A. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern. Med. 2013, 173, 1175–1185. [Google Scholar] [CrossRef]
- Singh, S.; Loke, Y.K.; Furberg, C.D. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. JAMA 2008, 300, 1439–1450. [Google Scholar] [CrossRef]
- Calverley, P.M.; Anderson, J.A.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Jones, P.W.; Crim, C.; Willits, L.R.; Yates, J.C.; Vestbo, J. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010, 65, 719–725. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Celli, B.; Senn, S.; Burkhart, D.; Kesten, S.; Menjoge, S.; Decramer, M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 1543–1554. [Google Scholar] [CrossRef]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S.; et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnacle, H.; Birk, R.; Brealey, N.; Locantore, N.; Lomas, D.A.; Ludwig-Sengpiel, A.; Mohindra, R.; Tabberer, M.; Zhu, C.-Q.; et al. FULFIL Trial: Once-Daily Triple Therapy for Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Kerwin, E.; Ayers, T.; Taylor, A.F.; Maitra, S.; Thach, C.; Lloyd, M.; Patalano, F.; Banerji, D. FLIGHT1 and FLIGHT2: Efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 192, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Rogliani, P.; Matera, M.G.; Cazzola, M. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 2016, 149, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Banerji, D.; Chapman, K.R.; Vestbo, J.; Roche, N.; Ayers, R.T.; Thach, C.; Fogel, R.; Patalano, F.; Vogelmeier, C.F.; et al. Indacaterol–glycopyrronium versus salmeterol–fluticasone for COPD. N. Engl. J. Med. 2016, 374, 2222–2234. [Google Scholar] [CrossRef]
- Stafyla, E.; Kotsiou, O.S.; Deskata, K.; Gourgoulianis, K.I. Missed diagnosis and overtreatment of COPD among smoking primary care population in Central Greece: Old problems persist. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 487–498. [Google Scholar] [CrossRef]
- Spyratos, D.; Chloros, D.; Michalopoulou, D.; Sichletidis, L. Estimating the extent and economic impact of under and overdiagnosis of chronic obstructive pulmonary disease in primary care. Chron. Respir. Dis. 2016, 13, 240–246. [Google Scholar] [CrossRef]
- Price, D.; West, D.; Brusselle, G.; Gruffydd-Jones, K.; Jones, R.; Miravitlles, M.; Rossi, A.; Hutton, C.; Ashton, V.L.; Stewart, R.; et al. Management of COPD in the UK primary-care setting: An analysis of real-life prescribing patterns. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 889–905. [Google Scholar] [CrossRef]
- Petite, S.E. Characterization of chronic obstructive pulmonary disease prescribing patterns in the United States. Pulm. Pharmacol. Ther. 2018, 49, 119–122. [Google Scholar] [CrossRef]
- Wei, Y.-F.; Kuo, P.-H.; Tsai, Y.-H.; Tao, C.-W.; Cheng, S.-L.; Lee, C.-H.; Wu, Y.-K.; Chen, N.-H.; Hsu, W.-H.; Hsu, J.-Y.; et al. Factors associated with the prescription of inhaled corticosteroids in GOLD group A and B patients with COPD–subgroup analysis of the Taiwan obstructive lung disease cohort. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1951–1956. [Google Scholar] [CrossRef][Green Version]
- Ivanov, Y.; Nikolaev, I.; Nemeth, I. Real-life evaluation of COPD treatment in a Bulgarian population: A 1-year prospective, observational, noninterventional study. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 653–663. [Google Scholar] [CrossRef]
- Desalu, O.O.; Onyedum, C.C.; Adeoti, A.O.; Gundiri, L.B.; Fadare, J.O.; Adekeye, K.A.; Onyeri, K.D.; Fawibe, A.E. Guideline-based COPD management in a resource-limited setting-physicians′ understanding, adherence, and barriers: A cross-sectional survey of internal and family medicine hospital-based physicians in Nigeria. Prim. Care Respir. J. 2013, 22, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.; Derom, E.; Liistro, G.; Marchand, E.; Ninane, V.; Peché, R.; Slabbynck, H.; Vincken, W.; Janssens, W. Overuse of inhaled corticosteroids in COPD: Five questions for withdrawal in daily practice. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Jebrak, G. COPD routine management in France: Are guidelines used in clinical practice? Rev. Mal. Respir. 2010, 27, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease, Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive of Pulmonary Disease. 2020 Report. Available online: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf (accessed on 9 May 2020).
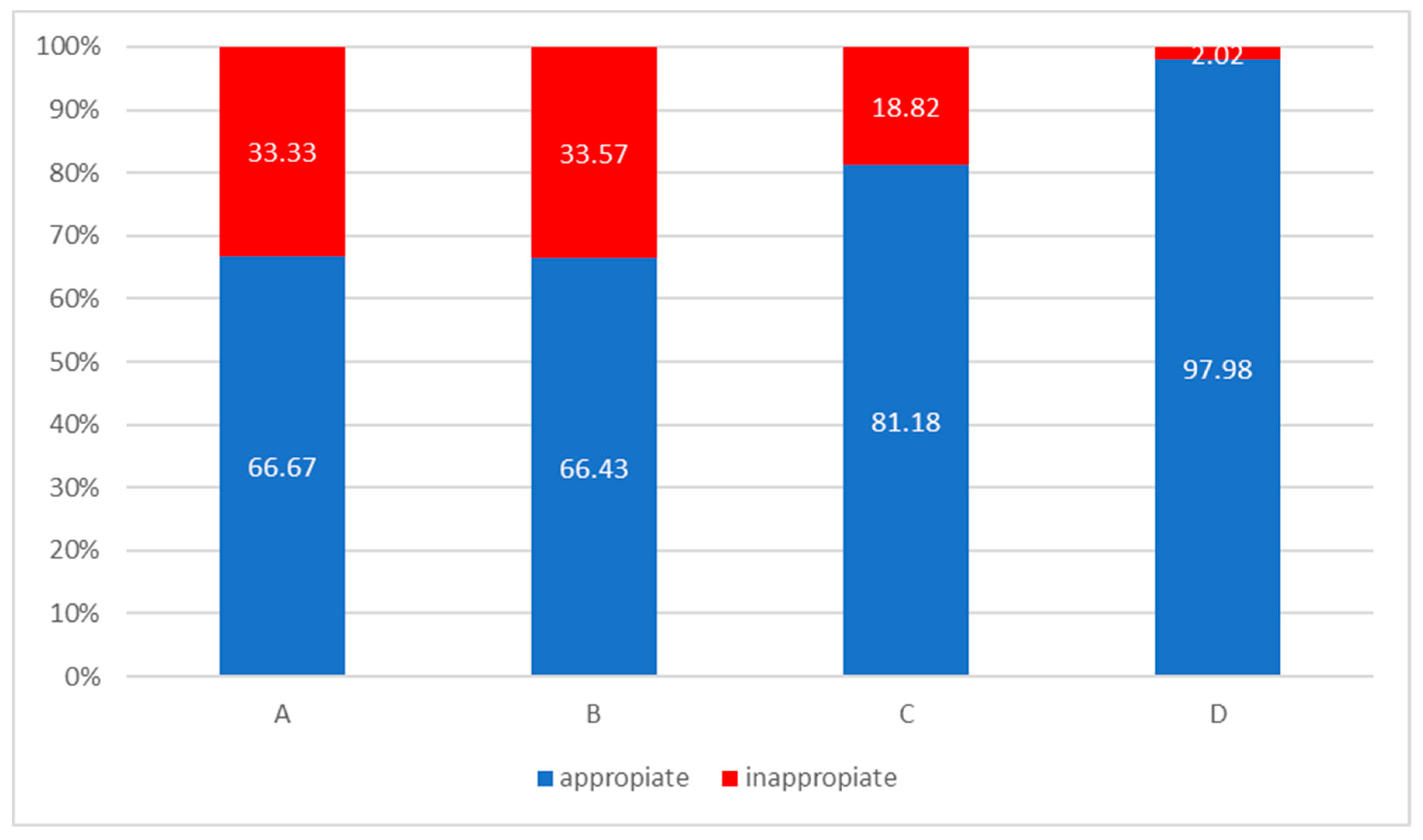
| Guideline-Concordant (Appropriate) | Guideline-Discordant (Inappropriate) | |||
|---|---|---|---|---|
| Group | First Choice | Alternative Choice | Undertreatment | Overtreatment |
| A | SABA or SAMA | Change the bronchodilator | No bronchodilator | LABA + LAMA, ICS-containing regimen |
| B | LAMA or LABA | LAMA + LABA | Only short-acting bronchodilator | ICS + LABA, ICS + LABA + LAMA, ICS + LAMA |
| C | LAMA | LAMA + LABA or ICS/LABA | Only ICS or LABA or SABA | ICS + LABA + LAMA, ICS + LAMA |
| D | LAMA + LABA or LAMA or ICS/LABA | If further exacerbations/symptoms ICS + LABA + LAMA | Only ICS or LABA or SABA, ICS + LAMA | |
| Variable | Frequency (n = 348) |
|---|---|
| Age (years, mean (SD)) | 68.61 (10.61) |
| Gender | |
| Male, n (%) | 252 (72.41) |
| Female, n (%) | 96 (27.59) |
| White race, n (%) | 348 (100) |
| Location of residency | |
| Urban | 191 (54.89) |
| Rural | 157 (45.11) |
| Smoking history, n (%) | 235 (67.53) |
| Current | 56 (23.83) |
| Former | 149 (63.40) |
| Never | 30 (12.77) |
| Pack-years (mean (SD)) (n = 117) | 40 (14.86) |
| Cardiovascular comorbidity, n (%) | 147 (42.24) |
| Chronic respiratory failure, n (%) | 115 (33.04) |
| Spirometric GOLD grade, n (%) | |
| GOLD 1 | 32 (9.20) |
| GOLD 2 | 132 (37.93) |
| GOLD 3 | 93 (26.72) |
| GOLD 4 | 91 (26.15) |
| GOLD group, n (%) | |
| A | 21 (6.03) |
| B | 143 (41.09) |
| C | 85 (24.43) |
| D | 99 (28.45) |
| Therapy | All | Group A | Group B | Group C | Group D |
|---|---|---|---|---|---|
| SABA, n (%) | 7 (2.01) | 5 (23.81) | 1 (0.7) | 0 (0) | 1 (1.01) |
| LABA, n (%) | 4 (1.15) | 0 (0) | 4 (2.8) | 0 (0) | 0 (0) |
| LAMA, n (%) | 66 (18.97) | 9 (42.86) | 43 (30.07) | 11 (12.94) | 3 (3.03) |
| LAMA + LABA, n (%) | 121 (34.77) | 6 (28.57) | 48 (33.57) | 41 (48.24) | 26 (26.26) |
| ICS, n (%) | 1 (0.29) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) |
| LABA + ICS, n (%) | 62 (17.82) | 1 (4.76) | 30 (20.98) | 17 (20) | 14 (14.14) |
| LAMA + LABA + ICS, n (%) | 84 (24.14) | 0 (0) | 14 (9.79) | 16 (18.82) | 54 (54.55) |
| No therapy | 3 (0.86) | 0 (0) | 2 (1.40) | 0 (0) | 1 (1.01) |
| Variable | Concordant (n = 275) | Discordant (n = 73) | p Value |
|---|---|---|---|
| Age (mean, (SD)) | 68.90 (10.31) | 67.53 (11.66) | 0.363 a |
| Male, n (%) | 201 (73.09) | 51 (69.86) | 0.583 b |
| Rural, n (%) | 130 (47.27) | 27 (36.98) | 0.116 b |
| Chronic respiratory failure, n (%) | 115 (41.82) | 31 (42.47) | 0.92 b |
| Cardiovascular comorbidities, n (%) | 147 (53.45) | 50 (68.49) | 0.02 b |
| First consultation, n (%) | 41 (14.91) | 10 (13.69) | 0.794 b |
| Groups A and B (n) vs. Groups C and D (n) | 109 vs. 166 | 55 vs. 18 | 0.0000001 b |
| Variable | First-Choice (n = 91) | Alternative-Choice (n = 184) | p Value |
|---|---|---|---|
| Age (mean, (SD)) | 67.65 (10.82) | 69.52 (10.02) | 0.171 a |
| Male, n (%) | 59 (64.83) | 142 (77.17) | 0.029 b |
| Rural, n (%) | 40 (43.96) | 90 (48.91) | 0.438 b |
| Chronic respiratory failure, n (%) | 23 (25.27) | 92 (50) | 0.00009 b |
| Cardiovascular comorbidities, n (%) | 147 (53.45) | 50 (68.49) | 0.869 b |
| First consultation, n (%) | 41 (14.91) | 10 (13.69) | 0.002 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajnoveanu, R.-M.; Rajnoveanu, A.-G.; Ardelean, A.-B.; Todea, D.A.; Pop, C.-M.; Antoniu, S.A.; Motoc, N.S.; Chis, A.F.; Fildan, A.P.; Man, M.A. Pulmonologists Adherence to the Chronic Obstructive Pulmonary Disease GOLD Guidelines: A Goal to Improve. Medicina 2020, 56, 422. https://doi.org/10.3390/medicina56090422
Rajnoveanu R-M, Rajnoveanu A-G, Ardelean A-B, Todea DA, Pop C-M, Antoniu SA, Motoc NS, Chis AF, Fildan AP, Man MA. Pulmonologists Adherence to the Chronic Obstructive Pulmonary Disease GOLD Guidelines: A Goal to Improve. Medicina. 2020; 56(9):422. https://doi.org/10.3390/medicina56090422
Chicago/Turabian StyleRajnoveanu, Ruxandra-Mioara, Armand-Gabriel Rajnoveanu, Andreea-Bianca Ardelean, Doina Adina Todea, Carmen-Monica Pop, Sabina Antonela Antoniu, Nicoleta Stefania Motoc, Ana Florica Chis, Ariadna Petronela Fildan, and Milena Adina Man. 2020. "Pulmonologists Adherence to the Chronic Obstructive Pulmonary Disease GOLD Guidelines: A Goal to Improve" Medicina 56, no. 9: 422. https://doi.org/10.3390/medicina56090422
APA StyleRajnoveanu, R.-M., Rajnoveanu, A.-G., Ardelean, A.-B., Todea, D. A., Pop, C.-M., Antoniu, S. A., Motoc, N. S., Chis, A. F., Fildan, A. P., & Man, M. A. (2020). Pulmonologists Adherence to the Chronic Obstructive Pulmonary Disease GOLD Guidelines: A Goal to Improve. Medicina, 56(9), 422. https://doi.org/10.3390/medicina56090422








