Rapid and Impressive Response to a Combined Treatment with Single-Dose Tocilizumab and NIV in a Patient with COVID-19 Pneumonia/ARDS
Abstract
1. Introduction
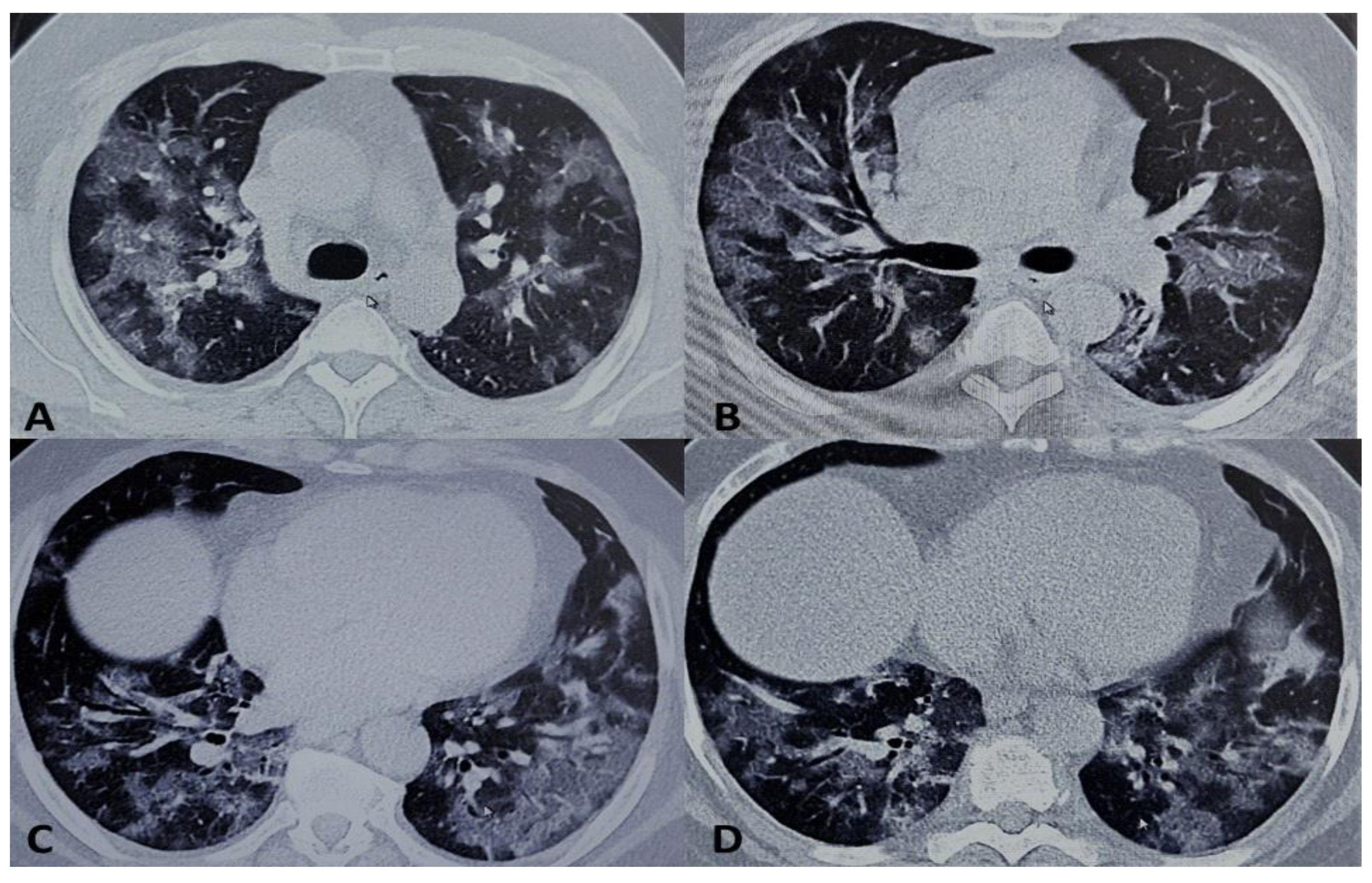
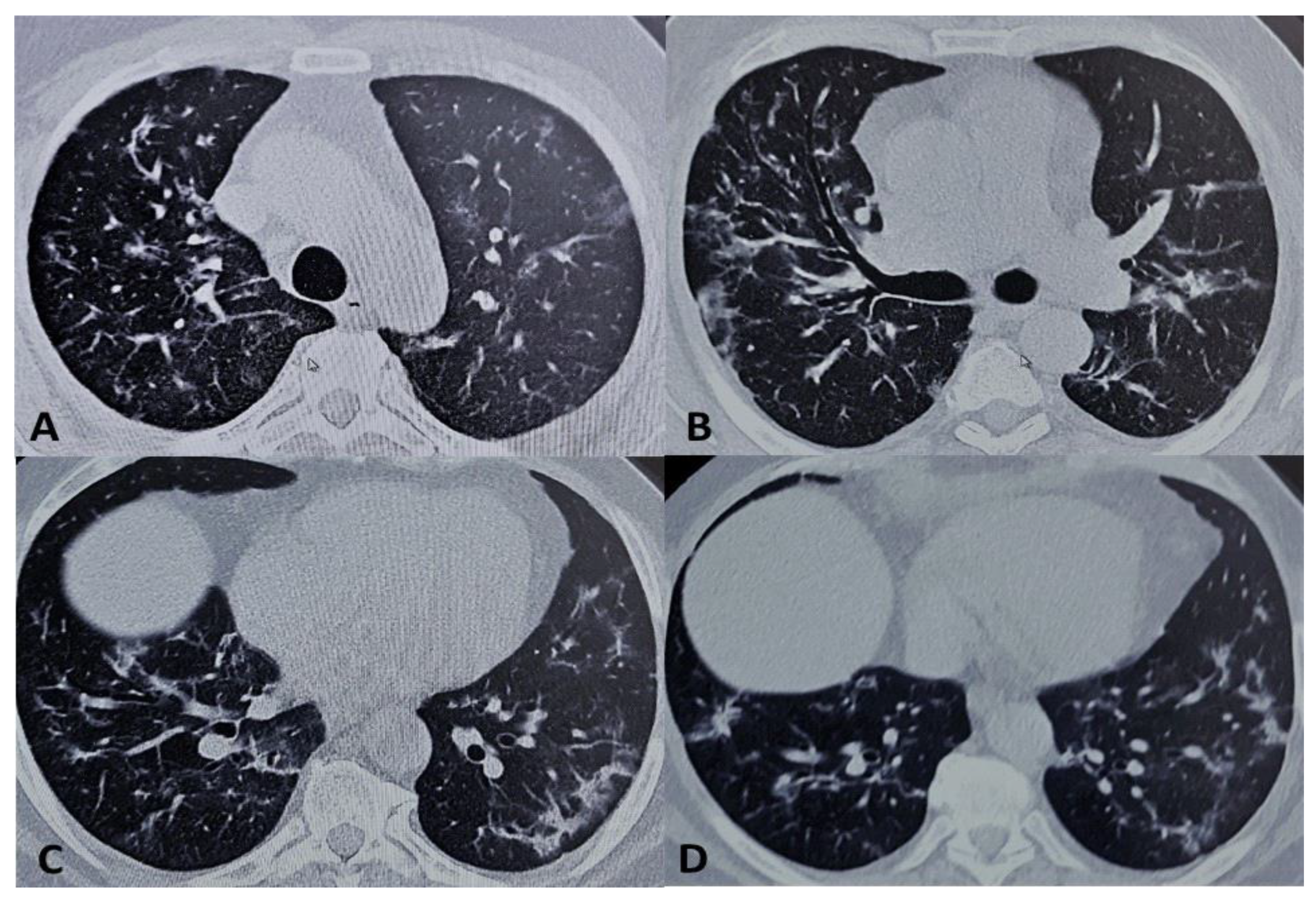
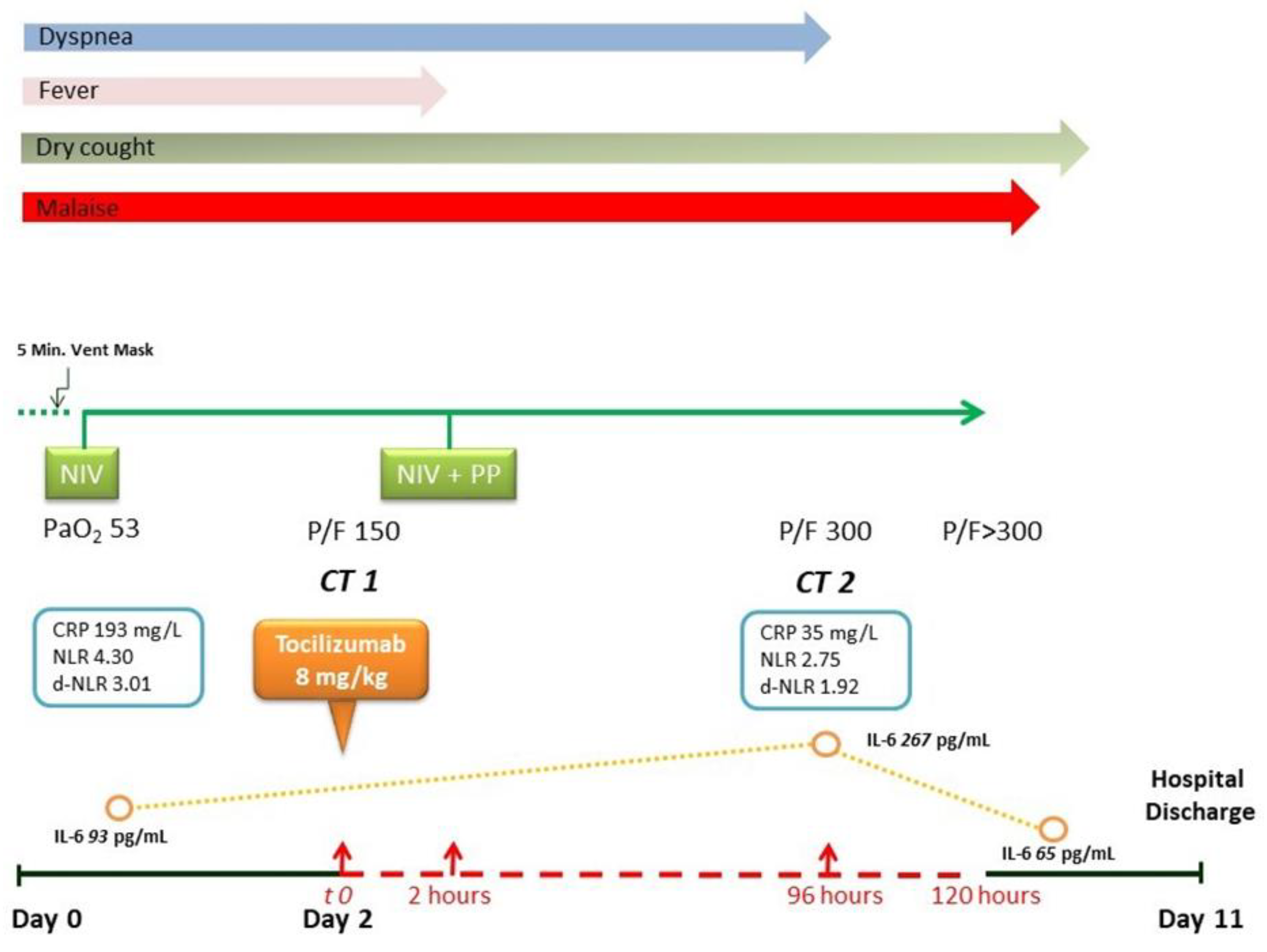
2. Presentation of Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 Pneumonia: Different Respiratory Treatments for Different Phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Meissner, K.; Marini, J.J. The Baby Lung and the COVID-19 Era. Intensive Care Med. 2020, 46, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Coppo, A.; Bellani, G.; Winterton, D.; Di Pierro, M.; Soria, A.; Faverio, P.; Cairo, M.; Mori, S.; Messinesi, G.; Contro, E.; et al. Feasibility and Physiological Effects of Prone Positioning in Non-Intubated Patients with Acute Respiratory Failure due to COVID-19 (Pron-COVID): A Prospective Cohort Study. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Sartini, C.; Tresoldi, M.; Scarpellini, M.; Tettamanti, A.; Carcò, F.; Landoni, G.; Zangrillo, M. Respiratory Parameters in Patients with COVID-19 after Using Noninvasive Ventilation in the Prone Position Outside the Intensive Care Unit. Jama 2020, 323, 2338–2340. [Google Scholar] [CrossRef]
- Chad, T.; Sampson, C. Prone Positioning in Conscious Patients on Medical Wards: A Review of the Evidence and its Relevance to Patients with COVID-19 Infection. Clin. Med. 2020, 20, e97–103. [Google Scholar] [CrossRef]
- Cao, W.; Li, T. COVID-19: Towards Understanding of Pathogenesis. Cell Res. 2020, 30, 367–369. [Google Scholar] [CrossRef]
- Lau, S.K.-P.; Lau, C.C.Y.; Chan, K.-H.; Li, C.P.Y.; Chen, H.; Jin, D.-Y.; Chan, J.F.-W.; Woo, P.C.Y.; Yuen, K. Delayed Induction of Proinflammatory Cytokines and Suppression of Innate Antiviral Response by the Novel Middle East Respiratory Syndrome Coronavirus: Implications for Pathogenesis and Treatment. J. Gen. Virol. 2013, 94 Pt 12, 2679–2690. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.Q. The Cytokine Release Syndrome (Crs) of Severe COVID-19 and Interleukin-6 Receptor (Il-6r) Antagonist Tocilizumab may be the Key to Reduce the Mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Fu, B.; Xu, X.; Wei, H. Why Tocilizumab could be an Effective Treatment for Severe COVID-19? J. Transl. Med. 2020, 18, 1–5. [Google Scholar] [CrossRef]
- Stroud, C.R.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the Management of Immune Mediated Adverse Events Secondary to PD-1 Blockade. J. Oncol. Pharm. Pract. 2019, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Ogata, A.; Kishimoto, T. A New Era for the Treatment of Inflammatory Autoimmune Diseases by Interleukin-6 Blockade Strategy. Semin. Immunol. 2014, 26, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. Jama 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Ding, L.; Wang, L.; Ma, W.; He, H. Efficacy and Safety of Early Prone Positioning Combined with HFNC or NIV in Moderate to Severe ARDS: A Multi-Center Prospective Cohort Study. Crit. Care 2020, 24, 28. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, F.; Beretta, L.; Scandroglio, A.M.; Colombo, S.; Landoni, G.; Ruggeri, A.; Peccatori, J.; D’Angelo, A.; De Cobelli, F.; Rovere-Querini, P.; et al. Microvascular COVID-19 Lung Vessels Obstructive Thromboinflammatory Syndrome (Microclots): An Atypical Acute Respiratory Distress Syndrome Working Hypothesis. Crit. Care Resusc. 2020. Available online: http://nsicu.ru/uploads/attachment/file/1011/ccr_landoni120_june_v6-2.pdf (accessed on 23 July 2020).
- Liu, B.; Li, M.; Zhou, Z.; Guan, X.; Xiang, Y. Can We Use Interleukin-6 (Il-6) Blockade For Coronavirus Disease 2019 (COVID-19)-Induced Cytokine Release Syndrome (CRS)? J. Autoimmun. 2020, 111, 102452. [Google Scholar] [CrossRef]
- Rojas-Marte, G.R.; Khalid, M.; Mukhtar, O.; Hashmi, A.T.; Waheed, M.A.; Ehrlich, S.; Aslam, A.; Siddiqui, S.; Agarwal, C.; Malyshev, Y.; et al. Outcomes in Patients with Severe COVID-19 Disease Treated with Tocilizumab. A Case-Controlled Study. Qjm Int. J. Med. 2020. [Google Scholar] [CrossRef]
- Quartuccio, L.; Sonaglia, A.; McGonagle, D.; Fabris, M.; Peghin, M.; Pecori, D.; de Monte, A.; Bove, T.; Curcio, F.; Bassi, F. Profiling COVID-19 Pneumonia Progressing into the Cytokine Stormsyndrome: Results from a Single Italian Centre Study on Tocilizumab Versusstandard of Care. J. Clin. Virol. 2020, 129, 104444. [Google Scholar] [CrossRef]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airò, P.; Bazzani, C.; Beindorf, E.-A.; et al. Tocilizumab for the Treatment of Severe COVID-19 Pneumonia with Hyperinflammatory Syndrome and Acute Respiratory Failure: A Single Center Study of 100 Patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef]
- Cellina, M.; Orsi, M.; Bombaci, F.; Sala, M.; Marino, P.; Oliva, G. Favorable Changes of CT Findings in a Patient with COVID-19 Pneumonia after Treatment with Tocilizumab. Diagn. Interv. Imaging 2020, 101, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Michot, M.; Albiges, L.; Chaput, N.; Saada, V.; Pommeret, F.; Griscelli, F.; Balleyguier, C.; Besse, B.; Marabelle, A.; Netzer, F. Tocilizumab, an Anti-IL6 Receptor Antibody, to Treat COVID-19-Related Respiratory Failure: A Case Report. Ann. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascella, M.; Mauro, I.; De Blasio, E.; Crispo, A.; Del Gaudio, A.; Bimonte, S.; Cuomo, A.; Ascierto, P.A. Rapid and Impressive Response to a Combined Treatment with Single-Dose Tocilizumab and NIV in a Patient with COVID-19 Pneumonia/ARDS. Medicina 2020, 56, 377. https://doi.org/10.3390/medicina56080377
Cascella M, Mauro I, De Blasio E, Crispo A, Del Gaudio A, Bimonte S, Cuomo A, Ascierto PA. Rapid and Impressive Response to a Combined Treatment with Single-Dose Tocilizumab and NIV in a Patient with COVID-19 Pneumonia/ARDS. Medicina. 2020; 56(8):377. https://doi.org/10.3390/medicina56080377
Chicago/Turabian StyleCascella, Marco, Immacolata Mauro, Elvio De Blasio, Anna Crispo, Alfredo Del Gaudio, Sabrina Bimonte, Arturo Cuomo, and Paolo Antonio Ascierto. 2020. "Rapid and Impressive Response to a Combined Treatment with Single-Dose Tocilizumab and NIV in a Patient with COVID-19 Pneumonia/ARDS" Medicina 56, no. 8: 377. https://doi.org/10.3390/medicina56080377
APA StyleCascella, M., Mauro, I., De Blasio, E., Crispo, A., Del Gaudio, A., Bimonte, S., Cuomo, A., & Ascierto, P. A. (2020). Rapid and Impressive Response to a Combined Treatment with Single-Dose Tocilizumab and NIV in a Patient with COVID-19 Pneumonia/ARDS. Medicina, 56(8), 377. https://doi.org/10.3390/medicina56080377





