Positron Emission Tomography-Based Response to Target and Immunotherapies in Oncology
Abstract
1. Introduction
2. From Tumor Shrinkage to Metabolic Response in Oncoematology: Methodological Overview
2.1. Metabolic Response in Solid Tumors
2.2. Metabolic Response in Lymphomas
3. PET Response to Target Therapies
4. PET to Response to Immunotherapy
4.1. Methodological Issues
4.2. PET-Based Response to ICPIs in Patients with Melanoma
4.3. PET-Based Response to ICPIs in Patients with NSCLC
5. Response Assessment and Prediction with NON-FDG Tracers
5.1. [11. C]choline, [18F]choline, and [68Ga]PSMA
5.2. [68. Ga]DOTA-Conjugate Peptides
5.3. Other Non-FDG Tracers
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jackson, S.E.; Chester, J.D. Personalised cancer medicine. Int. J. Cancer 2015, 137, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Haap, M.; Kopp, H.G.; Lipp, H.P. Tyrosine kinase inhibitors—A review on pharmacology, metabolism and side effects. Curr. Drug Metab. 2009, 10, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Rajkumar, S.V. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clin. Proc. 2015, 90, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Serkova, N.J.; Eckhardt, S.G. Metabolic Imaging to Assess Treatment Response to Cytotoxic and Cytostatic Agents. Front. Oncol. 2016, 6, 152. [Google Scholar] [CrossRef]
- O’Day, S.J.; Hamid, O.; Urba, W.J. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): A novel strategy for the treatment of melanoma and other malignancies. Cancer 2007, 110, 2614–2627. [Google Scholar] [CrossRef]
- Geukes Foppen, M.H.; Rozeman, E.A.; van Wilpe, S.; Postma, C.; Snaebjornsson, P.; van Thienen, J.V.; van Leerdam, M.E.; van den Heuvel, M.; Blank, C.U.; van Dieren, J.; et al. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open 2018, 3, e000278. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer. Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Aykan, N.F.; Özatlı, T. Objective response rate assessment in oncology: Current situation and future expectations. World J. Clin. Oncol. 2020, 11, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Min, S.J.; Jang, H.J.; Kim, J.H. Comparison of the RECIST and PERCIST criteria in solid tumors: A pooled analysis and review. Oncotarget 2016, 7, 27848–27854. [Google Scholar] [CrossRef] [PubMed]
- Odawara, S.; Kitajima, K.; Katsuura, T.; Kurahashi, Y.; Shinohara, H.; Yamakado, K. Tumor response to neoadjuvant chemotherapy in patients with esophageal cancer assessed with CT and FDG-PET/CT—RECIST 1.1 vs. PERCIST 1.0. Eur. J. Radiol. 2018, 101, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Belmouhand, M.; Löfgren, J.; Johannesen, H.H.; Baeksgaard, L.; Gutte, H.; Tariq, K.; Achiam, M.P. Early response evaluation of neoadjuvant therapy with PET/MRI to predict resectability in patients with adenocarcinoma of the esophagogastric junction. Abdom. Radiol. 2019, 44, 836–844. [Google Scholar] [CrossRef]
- Grootjans, W.; de Geus-Oei, L.F.; Troost, E.G.; Visser, E.P.; Oyen, W.J.; Bussink, J. PET in the management of locally advanced and metastatic NSCLC. Nat. Rev. Clin. Oncol. 2015, 12, 395–407. [Google Scholar] [CrossRef]
- Weber, W.A.; Petersen, V.; Schmidt, B.; Tyndale-Hines, L.; Link, T.; Peschel, C.; Schwaiger, M. Positron emission tomography in non-small-cell lung cancer: Prediction of response to chemotherapy by quantitative assessment of glucose use. J. Clin. Oncol. 2003, 21, 2651–2657. [Google Scholar] [CrossRef]
- Ding, Q.; Cheng, X.; Yang, L.; Zhang, Q.; Chen, J.; Li, T.; Shi, H. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST). J. Thorac. Dis. 2014, 6, 677–683. [Google Scholar]
- Pinker, K.; Riedl, C.; Weber, W.A. Evaluating tumor response with FDG PET: Updates on PERCIST, comparison with EORTC criteria and clues to future developments. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 55–66. [Google Scholar] [CrossRef]
- Kruse, V.; Mees, G.; Maes, A.; D’Asseler, Y.; Borms, M.; Cocquyt, V.; Van De Wiele, C. Reproducibility of FDG PET based metabolic tumor volume measurements and of their FDG distribution within. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 462–468. [Google Scholar] [PubMed]
- Shady, W.; Kishore, S.; Gavane, S.; Do, R.K.; Osborne, J.R.; Ulaner, G.A.; Gonen, M.; Ziv, E.; Boas, F.E.; Sofocleous, C.T. Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after (90)Y radioembolization of colorectal liver metastases: A comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur. J. Radiol. 2016, 85, 1224–1231. [Google Scholar] [CrossRef]
- Dosani, M.; Yang, R.; McLay, M.; Wilson, D.; Liu, M.; Yong-Hing, C.J.; Hamm, J.; Lund, C.R.; Olson, R.; Schellenberg, D. Metabolic tumour volume is prognostic in patients with non-small-cell lung cancer treated with stereotactic ablative radiotherapy. Curr. Oncol. 2019, 26, e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Nishio, M.; Nakanishi, H.; Hanai, N.; Hirakawa, H.; Kodaira, T.; Tamaki, T.; Hasegawa, Y. Impact of total lesion glycolysis measured by 18F-FDG-PET/CT on overall survival and distant metastasis in hypopharyngeal cancer. Oncol. Lett. 2016, 12, 1493–1500. [Google Scholar] [CrossRef]
- Skusa, C.; Weber, M.A.; Böttcher, S.; Thierfelder, K.M. Criteria-Based Imaging and Response Evaluation of Lymphoma 20 Years after Cheson: What is New?—A Review of the Current Classifications. Rofo 2020, 192, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Hutchings, M.; Loft, A.; Hansen, M.; Pedersen, L.M.; Buhl, T.; Jurlander, J.; Buus, S.; Keiding, S.; D’Amore, F.; Boesen, A.M.; et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 2006, 107, 52–59. [Google Scholar] [CrossRef]
- Gallamini, A.; Hutchings, M.; Rigacci, L.; Specht, L.; Merli, F.; Hansen, M.; Patti, C.; Loft, A.; Di Raimondo, F.; D’Amore, F. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J. Clin. Oncol. 2007, 25, 3746–3752. [Google Scholar] [CrossRef]
- Von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143. [Google Scholar] [CrossRef]
- Stroobants, S.; Goeminne, J.; Seegers, M.; Dimitrijevic, S.; Dupont, P.; Nuyts, J.; Martens, M.; van den Borne, B.; Cole, P.; Sciot, R.; et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur. J. Cancer 2003, 39, 2012–2020. [Google Scholar] [CrossRef]
- Sunaga, N.; Oriuchi, N.; Kaira, K.; Yanagitani, N.; Tomizawa, Y.; Hisada, T.; Ishizuka, T.; Endo, K.; Mori, M. Usefulness of FDG-PET for early prediction of the response to gefitinib in non-small cell lung cancer. Lung Cancer 2008, 59, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Bodenstein, C.; Dumont, R.A.; Seimbille, Y.; Dubinett, S.; Phelps, M.E.; Herschman, H.; Czernin, J.; Weber, W. Monitoring tumor glucose utilization by positron emission tomography for the prediction of treatment response to epidermal growth factor receptor kinase inhibitors. Clin. Cancer Res. 2006, 12, 5659–5667. [Google Scholar] [CrossRef] [PubMed]
- Aukema, T.S.; Kappers, I.; Olmos, R.A.; Codrington, H.E.; van Tinteren, H.; van Pel, R.; Klomp, H.M.; NEL Study Group. Is 18F-FDG PET/CT useful for the early prediction of histopathologic response to neoadjuvant erlotinib in patients with non-small cell lung cancer? J. Nucl. Med. 2010, 51, 1344–1348. [Google Scholar] [CrossRef]
- Benz, M.R.; Herrmann, K.; Walter, F.; Garon, E.B.; Reckamp, K.L.; Figlin, R.; Phelps, M.E.; Weber, W.A.; Czernin, J.; Allen-Auerbach, M.S. (18)F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. J. Nucl. Med. 2011, 52, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.S.; Park, H.L.; Yang, K.; Hwang, S.; Song, M.J.; Jang, J.W.; Choi, Y.W.; Yoon, S.K.; Yoo, I.R.; Bae, S.H. 18F-fluorodeoxyglucose uptake of hepatocellular carcinoma as a prognostic predictor in patients with sorafenib treatment. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Jacene, H.; Song, D.; Vilardell, F.; Messersmith, W.A.; Laheru, D.; Wahl, R.; Endres, C.; Jimeno, A.; Pomper, M.G.; et al. [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J. Clin. Oncol. 2009, 27, 2697–2704. [Google Scholar] [CrossRef]
- Beaver, J.A.; Hazarika, M.; Mulkey, F.; Mushti, S.; Chen, H.; He, K.; Sridhara, R.; Goldberg, K.B.; Chuk, M.K.; Chi, D.; et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2018, 19, 229–239. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Walunas, T.L.; Lenschow, D.J.; Bakker, C.Y.; Linsley, P.S.; Freeman, G.J.; Green, J.M.; Thompson, C.B.; Bluestone, J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994, 1, 405–413. [Google Scholar] [CrossRef]
- Giri, A.; Walia, S.S.; Gajra, A. Clinical Trials Investigating Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Rev. Recent Clin. Trials. 2016, 11, 297–305. [Google Scholar] [CrossRef]
- Ball, M.W.; Allaf, M.E.; Drake, C.G. Recent advances in immunotherapy for kidney cancer. Discov. Med. 2016, 21, 305–313. [Google Scholar]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef]
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Denoyelle, C.; Ohashi, P.S.; De Bono, J.S.; Mottaghy, F.M. Molecularly targeted therapies in cancer: A guide for the nuclear medicine physician. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Higuchi, T.; Naruse, I.; Arisaka, Y.; Tokue, A.; Altan, B.; Suda, S.; Mogi, A.; Shimizu, K.; Sunaga, N.; et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Lipson, E.J.; Im, H.J.; Rowe, S.P.; Gonzalez, E.M.; Blackford, A.; Chirindel, A.; Pardoll, D.M.; Topalian, S.L.; Wahl, R.L. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point 18F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J. Nucl. Med. 2017, 58, 1421–1428. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Larribere, L.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: Preliminary results of an ongoing study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 386–396. [Google Scholar] [CrossRef]
- Kong, B.Y.; Menzies, A.M.; Saunders, C.A.; Liniker, E.; Ramanujam, S.; Guminski, A.; Kefford, R.F.; Long, G.V.; Carlino, M.S. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment Cell Melanoma Res. 2016, 29, 572–577. [Google Scholar] [CrossRef]
- Dercle, L.; Ammari, S.; Seban, R.D.; Schwartz, L.H.; Houot, R.; Labaied, N.; Mokrane, F.Z.; Lazarovici, J.; Danu, A.; Marabelle, A. Kinetics and nadir of responses to immune checkpoint blockade by anti-PD1 in patients with classical Hodgkin lymphoma. Eur. J. Cancer 2018, 91, 136–144. [Google Scholar] [CrossRef]
- Ito, K.; Teng, R.; Schöder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. 18F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef]
- Rossi, G.; Bauckneht, M.; Genova, C.; Rijavec, E.; Biello, F.; Mennella, S.; Dal Bello, M.G.; Cittadini, G.; Bruzzi, P.; Piva, R.; et al. Comparison between 18F-FDG-PET- and CT-based criteria in non-small cell lung cancer (NSCLC) patients treated with Nivolumab [published online ahead of print, 2019 Dec 5]. J. Nucl. Med. 2020, 61, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Meignan, M. Current Evidence on PET Response Assessment to Immunotherapy in Lymphomas. PET Clin. 2020, 15, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Nobashi, T.; Baratto, L.; Reddy, S.A.; Srinivas, S.; Toriihara, A.; Hatami, N.; Yohannan, T.K.; Mittra, E. Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin. Nucl. Med. 2019, 44, e272–e279. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Dercle, L.; Lichtenstein, P.; Marabelle, A.; Michot, J.M.; Lambotte, O.; Le Pavec, J.; De Martin, E.; Balleyguier, C.; Champiat, S.; et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur. J. Cancer 2018, 96, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Pektor, S.; Hilscher, L.; Walzer, K.C.; Miederer, I.; Bausbacher, N.; Loquai, C.; Schreckenberger, M.; Sahin, U.; Diken, M.; Miederer, M. In vivo imaging of the immune response upon systemic RNA cancer vaccination by FDG-PET. EJNMMI Res. 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Decazes, P.; Bohn, P. Immunotherapy by Immune Checkpoint Inhibitors and Nuclear Medicine Imaging: Current and Future Applications. Cancers 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Emmett, L.; Lo, S.; Liu, V.; Kapoor, R.; Carlino, M.S.; Guminski, A.D.; Long, G.V.; Menzies, A.M. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann. Oncol. 2018, 29, 2115–2120. [Google Scholar] [CrossRef]
- Jreige, M.; Letovanec, I.; Chaba, K.; Renaud, S.; Rusakiewicz, S.; Cristina, V.; Peters, S.; Krueger, T.; de Leval, L.; Kandalaft, L.E.; et al. 18F-FDG PET metabolic-to-morphological volume ratio predicts PD-L1 tumour expression and response to PD-1 blockade in non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1859–1868. [Google Scholar] [CrossRef]
- Amrane, K.; Le Goupil, D.; Quere, G.; Delcroix, O.; Gouva, S.; Schick, U.; Salaun, P.Y.; Abgral, R.; Alavi, Z.; Keromnes, N.; et al. Prediction of response to immune checkpoint inhibitor therapy using 18F-FDG PET/CT in patients with melanoma. Medicine 2019, 98, e16417. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Mazziotti, E.; Toschi, L.; Lopci, E. Hyperprogressive Disease in Patients with Non-Small Cell Lung Cancer Treated with Checkpoint Inhibitors: The Role of 18F-FDG PET/CT. J. Nucl. Med. 2020, 61, 821–826. [Google Scholar] [CrossRef]
- Annovazzi, A.; Vari, S.; Giannarelli, D.; Pasqualoni, R.; Sciuto, R.; Carpano, S.; Cognetti, F.; Ferraresi, V. Comparison of 18F-FDG PET/CT Criteria for the Prediction of Therapy Response and Clinical Outcome in Patients With Metastatic Melanoma Treated With Ipilimumab and PD-1 Inhibitors. Clin. Nucl. Med. 2020, 45, 187–194. [Google Scholar] [CrossRef]
- Castello, A.; Carbone, F.G.; Rossi, S.; Monterisi, S.; Federico, D.; Toschi, L.; Lopci, E. Circulating Tumor Cells and Metabolic Parameters in NSCLC Patients Treated with Checkpoint Inhibitors. Cancers 2020, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kaira, K.; Yamaguchi, O.; Mouri, A.; Shiono, A.; Miura, Y.; Murayama, Y.; Kobayashi, K.; Kagamu, H.; Kuji, I. Potential of FDG-PET as Prognostic Significance after anti-PD-1 Antibody against Patients with Previously Treated Non-Small Cell Lung Cancer. J. Clin. Med. 2020, 9, 725. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.D.; Moya-Plana, A.; Antonios, L.; Yeh, R.; Marabelle, A.; Deutsch, E.; Schwartz, L.H.; Herrera Gómez, R.G.; Saenger, Y.; Robert, C.; et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, R.; Zaba, L.C.; Rosenberg, J.; Reddy, S.A.; Nobashi, T.W.; Davidzon, G.; Aparici, C.M.; Nguyen, J.; Moradi, F.; Iagaru, A.; et al. Prognostic value of volumetric PET parameters at early response evaluation in melanoma patients treated with immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Iravani, A.; Osman, M.M.; Weppler, A.M.; Wallace, R.; Galligan, A.; Lasocki, A.; Hunter, M.O.; Akhurst, T.; Hofman, M.S.; Lau, P.; et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Umeda, Y.; Morikawa, M.; Anzai, M.; Ameshima, S.; Kadowaki, M.; Waseda, Y.; Shigemi, H.; Tsujikawa, T.; Kiyono, Y.; Okazawa, H.; et al. Predictive value of integrated 18F-FDG PET/MRI in the early response to nivolumab in patients with previously treated non-small cell lung cancer. J. Immunother Cancer 2020, 8, e000349. [Google Scholar] [CrossRef]
- Castello, A.; Rossi, S.; Toschi, L.; Mansi, L.; Lopci, E. Soluble PD-L1 in NSCLC Patients Treated with Checkpoint Inhibitors and Its Correlation with Metabolic Parameters. Cancers 2020, 12, 1373. [Google Scholar] [CrossRef]
- De Giorgi, U.; Caroli, P.; Scarpi, E.; Conteduca, V.; Burgio, S.L.; Menna, C.; Moretti, A.; Galassi, R.; Rossi, L.; Amadori, D.; et al. 18F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1276–1283, Erratum in 2015, 42, 1337–1338. [Google Scholar] [CrossRef]
- De Giorgi, U.; Caroli, P.; Burgio, S.L.; Menna, C.; Conteduca, V.; Bianchi, E.; Fabbri, F.; Carretta, E.; Amadori, D.; Paganelli, G.; et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget 2014, 5, 12448–12458. [Google Scholar] [CrossRef]
- Maines, F.; Caffo, O.; Donner, D.; Sperduti, I.; Bria, E.; Veccia, A.; Chierichetti, F.; Tortora, G.; Galligioni, E. Serial 18F-choline-PET imaging in patients receiving enzalutamide for metastatic castration-resistant prostate cancer: Response assessment and imaging biomarkers. Future Oncol. 2016, 12, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Middendorp, M.; Maute, L.; Sauter, B.; Vogl, T.J.; Grünwald, F. Initial experience with 18F-fluoroethylcholine PET/CT in staging and monitoring therapy response of advanced renal cell carcinoma. Ann. Nucl. Med. 2010, 24, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Yamamoto, S.; Nakanishi, Y.; Yamada, Y.; Hashimoto, T.; Suzuki, T.; Go, S.; Kanematsu, A.; Nojima, M.; Fujiwara, M.; et al. Evaluation of Treatment Response in Prostate Cancer and Renal Cell Carcinoma Patients Using 11C-choline PET/CT Findings. Acta. Med. Okayama 2019, 73, 341–347. [Google Scholar] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Dorff, T.B.; Fanti, S.; Farolfi, A.; Reiter, R.E.; Sadun, T.Y.; Sartor, O. The Evolving Role of Prostate-Specific Membrane Antigen-Based Diagnostics and Therapeutics in Prostate Cancer. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Castellucci, P.; Fanti, S. Current application and future perspectives of prostate specific membrane antigen PET imaging in prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 7–18. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Pillai, A.M.; Knapp, F.F., Jr. Lutetium-177 Labeled Therapeutics: ¹⁷⁷Lu-PSMA is Set to Redefine Prostate Cancer Treatment. Curr. Radiopharm. 2016, 9, 6–7. [Google Scholar] [CrossRef]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a Prospective Phase 2 Pilot Trial of 177Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601, Erratum in 2017, 44, 2150–2151. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisio, T.S.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816, Erratum in O’Dorisio, T.M. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 584. [Google Scholar] [CrossRef]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mammatas, L.H.; Venema, C.M.; Schröder, C.P.; de Vet, H.C.W.; van Kruchten, M.; Glaudemans, A.W.J.M.; Yaqub, M.M.; Verheul, H.M.W.; Boven, E.; van der Vegt, B.; et al. Visual and quantitative evaluation of [18F]FES and [18F]FDHT PET in patients with metastatic breast cancer: An interobserver variability study. EJNMMI Res. 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Venema, C.M.; Mammatas, L.H.; Schröder, C.P.; van Kruchten, M.; Apollonio, G.; Glaudemans, A.W.J.M.; Bongaerts, A.H.H.; Hoekstra, O.S.; Verheul, H.M.W.; Boven, E.; et al. Androgen and Estrogen Receptor Imaging in Metastatic Breast Cancer Patients as a Surrogate for Tissue Biopsies. J. Nucl. Med. 2017, 58, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Ahn, S.H.; Kim, S.B.; Han, S.; Lee, S.H.; Oh, S.J.; Lee, S.J.; Kim, H.J.; Ko, B.S.; Lee, J.W.; et al. Diagnostic accuracy and safety of 16α-[18F]fluoro-17β-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: A prospective cohort study. Lancet Oncol. 2019, 20, 546–555. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.A. The quest for improving the management of breast cancer by functional imaging: The discovery and development of 16α-[18F]fluoroestradiol (FES), a PET radiotracer for the estrogen receptor, a historical review. Nucl. Med. Biol. 2020. [Google Scholar] [CrossRef]
- Kurland, B.F.; Peterson, L.M.; Lee, J.H.; Linden, H.M.; Schubert, E.K.; Dunnwald, L.K.; Link, J.M.; Krohn, K.A.; Mankoff, D.A. Between-patient and within-patient (site-to-site) variability in estrogen receptor binding, measured in vivo by 18F-fluoroestradiol PET. J. Nucl. Med. 2011, 52, 1541–1549. [Google Scholar] [CrossRef]
- Nienhuis, H.H.; van Kruchten, M.; Elias, S.G.; Glaudemans, A.W.J.M.; de Vries, E.F.J.; Bongaerts, A.H.H.; Schröder, C.P.; de Vries, E.G.E.; Hospers, G.A.P. 18F-Fluoroestradiol Tumor Uptake Is Heterogeneous and Influenced by Site of Metastasis in Breast Cancer Patients. J. Nucl. Med. 2018, 59, 1212–1218. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Dehdashti, F.; Siegel, B.A.; Katzenellenbogen, J.A.; Fracasso, P.; Welch, M.J. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: Correlation with estrogen receptor status and response to systemic therapy. Clin. Cancer Res. 1996, 2, 933–939. [Google Scholar]
- Van Kruchten, M.; de Vries, E.G.; Glaudemans, A.W.; van Lanschot, M.C.; van Faassen, M.; Kema, I.P.; Brown, M.; Schröder, C.P.; de Vries, E.F.; Hospers, G.A. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015, 5, 72–81. [Google Scholar] [CrossRef]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef]
- Linden, H.M.; Kurland, B.F.; Peterson, L.M.; Schubert, E.K.; Gralow, J.R.; Specht, J.M.; Ellis, G.K.; Lawton, T.J.; Livingston, R.B.; Petra, P.H. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin. Cancer Res. 2011, 17, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.A.M.S.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
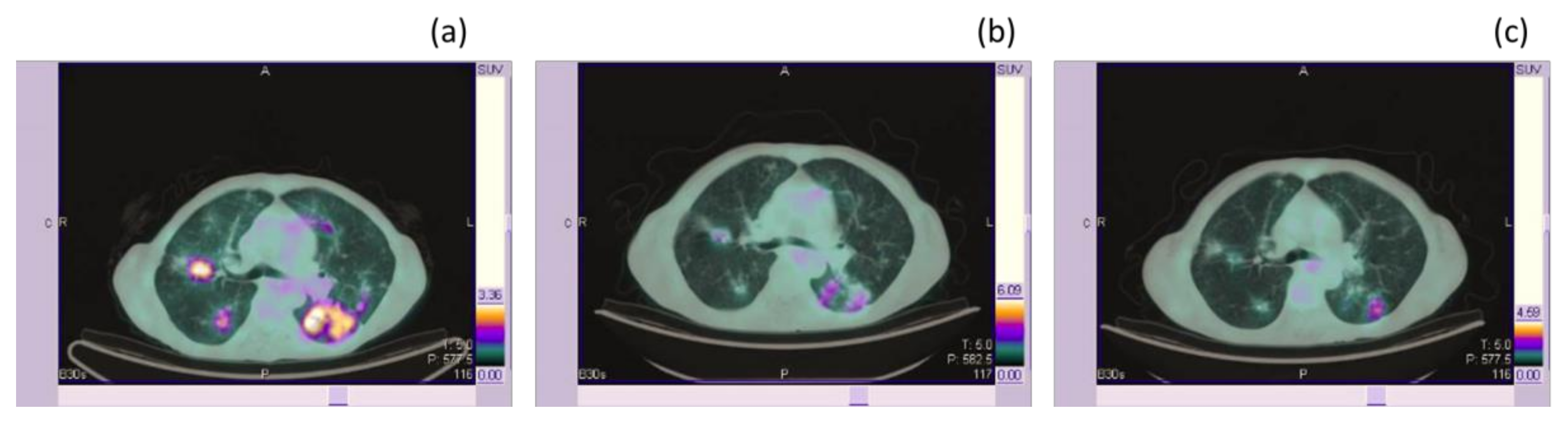
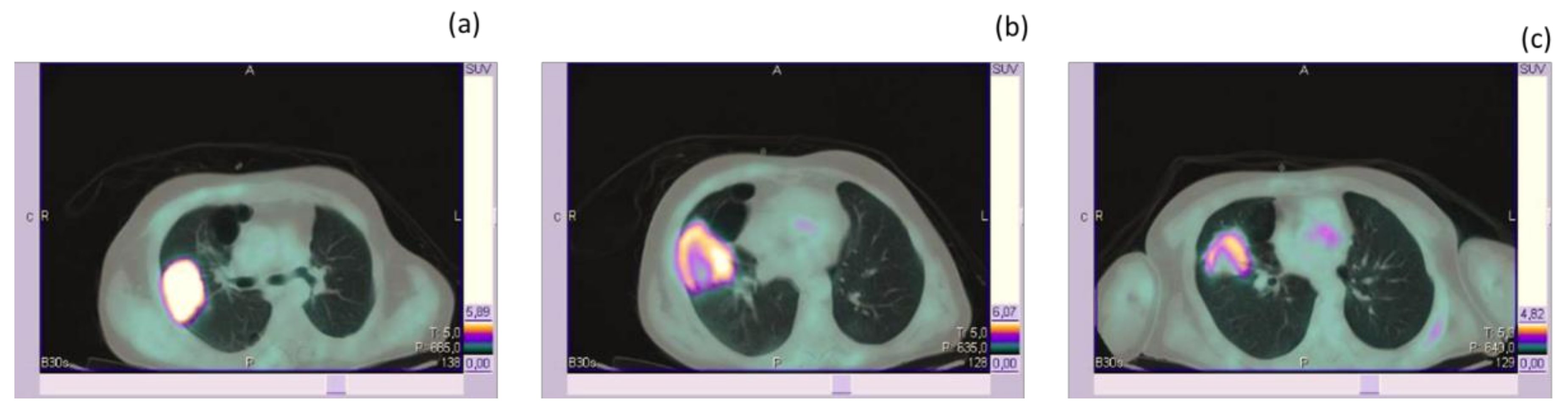
| Category | RECIST 1.1 | EORTC (1999) | PERCIST | LUGANO |
|---|---|---|---|---|
| Target lesion | Up 2 per organs, maximum 5 in total | The most [18F]FDG avid lesions (SUV BSA). Number of lesions not specified | The hottest single tumor lesion at baseline [18F]FDG PET (SUL peak) |
|
| New lesion | Any new lesion results in progressive disease at first appearance | |||
| Complete response |
| Complete absence of [18F]FDG uptake | Complete resolution of [18F]FDG uptake within the target lesion (< mean liver activity and indistinguishable from background/blood pool and no new [18F]FDG avid lesions) | DS 1, 2, and 3 in nodal or extra-nodal sites, with or without residual mass |
| Partial response |
| A decrease in SUV > 25% | A reduction of a minimum of 30% in the target tumor [18F]FDG SUL peak PMR | DS 4 or 5 with [18F]FDG uptake decreased compared with baseline |
| Progressive disease |
| An increase in SUV > 25% or appearance of new lesions | A 30% increase in [18F]FDG SUL peak or advent of new [18F]FDG avid lesions | DS 4 or 5 with an increase in uptake from baseline &/or new lesions |
| Stable disease | Neither partial response nor progressive disease | Increase in SUV by < 25% or decrease in SUV by < 15% | Disease other than CMR, PMR or PMD | DS 4 or 5 with no change in [18F]FDG uptake |
| Reference | Study Type | Patients’ Characteristics | Aims | Methods | Results | Conclusions |
|---|---|---|---|---|---|---|
| Sachpekidis et al. [47] | prospective | 22 patients suffering from unresectable metastatic melanoma, scheduled for ipilimumab treatment. | To evaluate the role of [18F]FDG PET/CT performed after two cycles of ipilimumab in predicting the final response to therapy. | PET/CT scanning was performed before the start of treatment (baseline scan), after two cycles of treatment (early response) and at the end of treatment after four cycles (late response). Evaluation of the patient response to treatment on PET was based on EORTC criteria. | Early PET/CT performed after two ipilimumab cycles predicted treatment response in 13 of the 15 PMD patients, in five of the five SMD patients and in neither of the two PMR patients. | [18F]FDG PET/CT after two cycles of ipilimumab is highly predictive of the final treatment outcome in patients with PMD and SMD. |
| Kong et al. [48] | prospective | 27 patients with unresectable stage IIIC or IV melanoma after prolonged treatment with anti-PD-1 antibodies. | To examine the hypothesis that patients with prolonged response to treatment may have metabolically inactive lesions by [18F]FDG PET/CT. | Scans were performed at a median of 15.2 months (range 12-29 months) after starting treatment. | 8 patients with positive scans underwent biopsy; 5 of 8 (62%) were melanoma and 3 of 8 (38%) were immune cell infiltrates. Of the 12 patients with negative [18F]FDG PET scans, 6 had residual computerized tomography-visible lesions, 5 have ceased treatment, and none have recurred with follow-up of 6-10 months. | Patients with residual metastases after a prolonged period without progression on anti-PD-1 therapy may have metabolically inactive lesions. Isolated metabolically active lesions in clinically well patients may reveal immune cell infiltrates rather than melanoma. |
| Cho et al. [46] | prospective | 20 patients with advanced melanoma receiving ICIPs. | To evaluate [18F]FDG PET/CT scanning as an early predictor of response to immune checkpoint inhibitors in patients with advanced melanoma. | [18F]FDG PET/CT was performed at 3 scan intervals. Tumor response at each posttreatment time point was assessed according to RECIST 1.1, PERCIST 1.0 and EORTC criteria. | Early response evaluations using RECIST 1.1, immune-related response criteria, PERCIST, and EORTC criteria demonstrated accuracies of 75%, 70%, 70%, and 65%, respectively. By combining early anatomic and functional imaging data criteria to predict eventual response were developed. | Combining functional and anatomic imaging parameters from [18F]FDG PET/CT scans performed early during immunotherapy appears predictive for eventual response in patients with advanced melanoma. |
| Dercle et al. [49] | retrospective | 16 heavily pre-treated patients with Hodgkin’s Lymphoma (HL). | To define the depth and time of maximal anti-tumor response to anti-PD1 in heavily pre-treated patients with HL. | The [18F]FDG PET/CT and CT data of all relapsed or refractory HL were reviewed according to the International Harmonisation Project Cheson 2014 criteria and the LYRIC criteria. | Fifty-six percent of patients (9/16) achieved an objective response at 3 months, including 19% (3/16) of complete response. Seventeen percent (1/6) of partial responders at 3 months were converted in a complete response. 22% (2/9) of responders at 3 months relapsed before one year. The nadir was reached at 12.7 (3.0-23.0) months. The median (range) depth of response at nadir was -77% (-50% to 100%). | Complete metabolic responses occurred within 6 months, a minority of partial responses were converted in complete response, and the median nadir was observed one year after treatment initiation. These data could help to better define the optimal treatment strategy by PET or CECT-directed approaches |
| Tan et al. [57] | retrospective | 140 metastatic melanoma patients treated with anti-PD-1-based immunotherapy with baseline and 1-year [18F]FDG PET and CT imaging. | To investigate whether [18F]FDG PET may better predict long-term outcomes compared with CT | One-year response was determined using RECIST for CT and EORTC criteria for PET. PFS was determined from the 1-year landmark. | Whilst only a small proportion of patients have a CR at 1 year, most patients with a PR have CMR on PET. Almost all patients with CMR at 1 year have ongoing response to therapy thereafter. | PET may have utility in predicting long-term benefit and help guide discontinuation of therapy. |
| Ito et al. [50] | retrospective | 60 patients with metastatic melanoma who underwent [18F]FDG PET/CT scans both before and after ipilimumab therapy. | To evaluate the association between tumor response on [18F]FDG PET/CT and prognosis in patients with metastatic malignant melanoma treated with ipilimumab. | Tumor response was assessed by the change in the sum of SULpeak of up to 5 lesions according to PERCIST5. New lesions on PET that appeared suggestive of metastases were considered PMD. An immunotherapy-modified response classification was also evaluated (imPERCIST5). | In responders and non-responders, the 2-y OS was 66% versus 29% for imPERCIST5 (p = 0.003). After multivariate analysis, imPERCIST5 remained prognostic (p = 0.005). New sites of focal [18F]FDG uptake occurred more often in patients with PMD (n = 24) by imPERCIST5 than in those with stable metabolic disease (n = 7) or partial metabolic response (n = 4). | In patients with metastatic melanoma treated with ipilimumab, tumor response according to PERCIST was associated with OS. Our data suggest that PMD should not be defined by the appearance of new lesions, but rather by an increase in the sum of SULpeak. |
| Jreige et al. [58]] | retrospective | 49 patients with confirmed NSCLC. | To investigate correlation between [18F]FDG PET/CT-based markers and tumor tissue expression of PD-L1, necrosis and clinical outcome in patients treated with ICPIs | SUVmax, SUVmean, MTV and TLG were obtained from [18F]FDG PET/CT images. Metabolic-to-morphological volume ratio (MMVR) was measured. | All tumors showed metabolic [18F]FDG PET uptake. MMVR was correlated inversely with PD-L1 expression in tumor cells. Furthermore, PD-L1 expression and low MMVR were significantly correlated with clinical benefit. Necrosis was correlated negatively with MMVR. | MMVR was introduced as a new imaging biomarker and its ability to noninvasively capture increased PD-L1 tumor expression and predict clinical benefit from checkpoint blockade in NSCLC should be further evaluated. |
| Amrane et al. [59] | retrospective | 37 patients with unresectable metastatic cutaneous melanoma eligible for immunotherapy. | To assess serial [18F]FDG PET/CT imaging according to morphological and functional to predict clinical response to therapy in patients with advanced melanoma receiving immune checkpoint blocking agents. | Among 37 assessed patients, 27 had 1 line of ICI, 8 had 2 lines of ICI and 2 patients had 3 lines of ICI: total of 49 PET/CTs. | Median PFS was 29.62 months (p = 0.001: RECIST 1.1), (p < 0.0001: iRECIST), (p = 0.000: PERCIST), (p = 0.072: PECRIT). Median OS was 36.62 months (p = 0.005: RECIST 1.1), (p < 0.0001: iRECIST), (p = 0.001: PERCIST), (p = 0.082 PECRIT). | [18F]FDG PET/CT scans could detect eventual ICI-response in patients with metastatic melanoma. According to our study, iRECIST and PERCIST 1.0 may provide the most optimal ICI-related response classification. |
| Rossi et al. [51] | prospective | 72 patients with advanced NSCLC. | To compare the evaluation of first response to Nivolumab by means of CT-based criteria with respect to [18F]FDG PET response criteria in NSCLC patients. | Patients underwent CT scan and FDG-PET at baseline and after 4 cycles (first evaluation). Response was evaluated with CT scan by means RECIST 1.1 and IrRC and with FDG-PET by means of PERCIST and imPERCIST criteria. The concordance between CT- and PET-based criteria and the capability of each method to OS were evaluated. | A low concordance between CT- and PET-based criteria was observed. Looking at OS, IrRC were more reliable to distinguish responders from non-responders. However, thanks to the prognostic value of partial metabolic response assessed by both PERCIST and Immuno-PERCIST, PET-based response maintained prognostic significant in patients classified as progressive disease on the basis of IrRC. | The added prognostic value of the metabolic response assessment, potentially improving the therapeutic decision-making was suggested. |
| Castello et al. [60] | prospective | 50 NSCLC patients treated with ICIs. | To investigate the prevalence of such a phenomenon and to assess its association with clinical variables and metabolic parameters by [18F]FDG PET/CT. | All patients underwent contrast-enhanced CT, [18F]FDG PET/CT, and complete peripheral blood sampling at baseline before ICI treatment. A Cox proportional hazards regression analysis was used to evaluate factors independently associated with OS. | Survival analysis showed a median OS of 4 months for the HPD group, compared with 15 mo for the non-HPD group (p = 0.003). Median OS was significantly different when all the response categories were considered. Multivariate analysis identified MTV and derived neutrophil-to-lymphocyte ratio as independent predictors for OS. | The use of ICIs might represent a concern in patients with high metabolic tumor burden and inflammatory indices at baseline. |
| Annovazzi et al. [61] | retrospective | 57 patients with metastatic melanoma treated with ipilimumab or with PD-1 inhibitors who performed an [18F]FDG PET/CT scan before treatment and 12 to 18 weeks later. | To compare the diagnostic accuracy of different [18F]FDG PET/CT criteria to predict therapy response and clinical outcome in melanoma patients treated with immune checkpoint inhibitors. | Response at PET1 was evaluated according to RECIST 1.1, EORTC, PERCIMT, and by percentage change of (MTV) and TLG of up to 5 target lesions. Performance of each criterion at PET1 to predict clinical benefit at 6 months since starting immunotherapy was assessed and correlated to PFS. | The best predictor of therapy response was MTV combined with PERCIMT criteria (accuracy, 0.96). In group 2, overlapping results were found for EORTC, MTV, and total lesion glycolysis (accuracy, 0.97). The reliability of the above parameters was also confirmed in the progression-free survival analysis. | [18F]FDG PET/CT performed after 3 to 4 months since starting immunotherapy can correctly evaluate response to treatment and can also able to predict long-term clinical outcome. Performance of [18F]FDG PET/CT and criteria for response assessment is influenced by the class of treatment. |
| Castello et al. [62] | prospective | 35 NSCLC patients | To examine CTC count and its association with metabolic parameters and clinical outcomes in NSCLC patients treated with ICI. | All patients underwent an [18F]FDG PET/CT scan and CTC detection through Isolation by Size of Tumor/Trophoblastic Cells (ISET) from peripheral blood samples obtained at baseline and 8 weeks after ICI initiation. Association of CTC count with clinical and metabolic characteristics was studied. | ΔCTC was significantly associated with tumor metabolic response set by EORTC criteria (p = 0.033). At the first restaging, patients with a high tumor burden, that is, metabolic tumor volume (MTV) and total lesion glycolysis (TLG), had a higher CTC count (p = 0.009). Multivariate analysis identified CTC count at 8 weeks as an independent predictor for PFS and OS, whereas ΔMTV and maximum standardized uptake value variation (ΔSUVmax) was predictive for PFS and OS, respectively. | CTC number is modulated by previous treatments and correlates with metabolic response during ICI. Moreover, elevated CTC count, along with metabolic parameters, are prognostic factors for PFS and OS. |
| Hashimoto et al. [63] | retrospective | 85 patients with previously treated NSCLC who underwent [18F]FDG PET just before administration of nivolumab or pembrolizumab. | To retrospectively examine the prognostic significance of [18F]FDG uptake as a predictive marker of anti-PD-1 antibody. | MTV, TLG and SUVmax on [18F]FDG uptake were assessed. | The tumor metabolic activity by TLG and MTV was identified as an independent prognostic factor for predicting outcome after anti-PD-1 antibody therapy. | TLG and MTV on [18F]FDG uptake may predict the prognosis after anti-PD-1 antibodies in patients with previously treated NSCLC. |
| Seban et al. [64] | retrospective | 56 patients with non-resectable mucosal melanoma (Muc-M) or cutaneous melanoma (Cut-M) who underwent baseline [18F]FDG PET/CT before treatment with ICIs. | To compare the prognostic value of imaging biomarkers derived from a quantitative analysis of baseline [18F]FDG PET/CT in patients with mucosal melanoma (Muc-M) or cutaneous melanoma (Cut-M) treated with ICIs. | Parameters were extracted from (i) tumoral tissues: SUVmax, SUVmean, TMTV and TLG and (ii) lymphoid tissues: BLR and SLR. Association with survival and response was evaluated using Cox prediction models, | In Muc-M, increased tumor SUVmax was associated with shorter OS while it was not correlated with PFS, ORR, or DCR. In Cut-M, increased TMTV and increased BLR were independently associated with shorter OS, shorter PFS, and lower response (ORR, DCR). | For Muc-M patients treated with ICI, the only prognostic imaging biomarker was a high baseline maximal glycolytic activity (SUVmax), whereas for Cut-M patients, baseline metabolic tumor burden or bone marrow metabolism was negatively correlated to ICI response duration. |
| Nakamoto et al. [65] | retrospective | 85 melanoma patients treated with ICIs who underwent PET/CT scans before and approximately 3 months after the start of immunotherapy. | To investigate the prognostic value of MTV and other metabolic tumor parameters, obtained from baseline and first restaging [18F]FDG PET/CT scans in melanoma patients treated with ICIs. | Metabolic tumor parameters including MTV for all melanoma lesions were measured on each scan. A Cox proportional hazards model was used for univariate and multivariate analyses of metabolic parameters combined with known clinical prognostic factors associated OS. | MTV obtained from first restaging PET/CT scans (MTVpost) was the strongest prognostic factor for OS among PET/CT parameters (p < 0.0001). The median OS in patients with high MTVpost (≥ 23.44) was 16 months as compared with more than 60 months in patients with low MTVpost (p = 0.0003). | Whole-body metabolic tumor volume from PET scan acquired approximately 3 months following initiation of immunotherapy (MTVpost) is a strong prognostic indicator of OS in melanoma patients. |
| Iravani et al. [66] | retrospective | 31 patients who had first-line nivolumab plus ipilimumab; pre- and post-treatment [18F]FDG PET/CT scans within 2 and 4 months of starting ICI, respectively and at least one lesion assessable by PERCIST. | To investigate the role of [18F]FDG PET/CT in monitoring of response and immune-related adverse events following first-line combination-ICI therapy for advanced melanoma. | Outcomes in patients who had first-line nivolumab plus ipilimumab were reviewed; pre- and post-treatment FDG-PET/CT scans within 2 and 4 months of starting ICI, respectively; and at least one lesion assessable by PERCIST. | The best-overall responses were CMR in 25 (80%), PMR in 3 (10%), and PMD in 3 (10%) patients. Patients with PMD had significantly higher pre-treatment wbMTV (p = 0.009). Secondary progression The most common [18F]FDG PET/CT detectable immune-related adverse event were endocrinopathies and enterocolitis. | [18F]FDG PET/CT response evaluation predicts the long-term outcome of patients treated with first-line combination-ICIs.. Beyond response assessment, [18F]FDG PET/CT frequently detects clinically relevant irAEs. |
| Umeda et al. [67] | prospective | 25 with previously treated NSCLC | To determine whether changes in integrated [18F]FDG PET/MRI parameters after the first 2 weeks of antiprogrammed death-1 antibody nivolumab therapy could predict the response of patients with NSCLC. | Patients underwent [18F]FDG PET/MRI before and at 2 weeks after nivolumab therapy. Changes in SUVmax, ΔTLG and ΔADC between the two scans were calculated and evaluated for their associations with the clinical response to therapy. | Non-PD patients had significantly decreased TLG, increased ADCmean and lower ΔTLG + ΔADCmean than PD patients. | A combination of ΔTLG and ΔADCmean measured by integrated [18F]FDG PET/MRI may have value as a predictor of the response and survival of patients with NSCLC following nivolumab therapy. |
| Castello et al. [68] | prospective | 20 NSCLC patients candidate to ICI therapy. | To investigate the role of sPD-L1 in NSCLC patients treated with ICI and to analyze its association with clinical outcomes and metabolic parameters by [18F]FDG PE T/CT. | Patients who had serum frozen samples and [18F]FDG PET/CT available, both at baseline and at the first restaging after approximately three or four cycles of ICI, were included. Before and after 3–4 cycles of ICI, peripheral blood samples were collected from patients. | A significant association between patients with elevated sPD-L1, above the median value, and high metabolic tumor burden, expressed by MTV (p = 0.034) and TLG (p = 0.049) was found. At the first restaging after 7–8 weeks, median sPD-L1 levels significantly increased as compared to baseline median value (p = 0.017). | The association between metabolic tumor burden and sPD-L1 levels, as well as a significant increase of sPD-L1 during treatment with ICI were demonstrated. PD-L1 can be used as a new biomarker in the early assessment and monitoring of immunotherapy efficacy. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donegani, M.I.; Ferrarazzo, G.; Marra, S.; Miceli, A.; Raffa, S.; Bauckneht, M.; Morbelli, S. Positron Emission Tomography-Based Response to Target and Immunotherapies in Oncology. Medicina 2020, 56, 373. https://doi.org/10.3390/medicina56080373
Donegani MI, Ferrarazzo G, Marra S, Miceli A, Raffa S, Bauckneht M, Morbelli S. Positron Emission Tomography-Based Response to Target and Immunotherapies in Oncology. Medicina. 2020; 56(8):373. https://doi.org/10.3390/medicina56080373
Chicago/Turabian StyleDonegani, Maria Isabella, Giulia Ferrarazzo, Stefano Marra, Alberto Miceli, Stefano Raffa, Matteo Bauckneht, and Silvia Morbelli. 2020. "Positron Emission Tomography-Based Response to Target and Immunotherapies in Oncology" Medicina 56, no. 8: 373. https://doi.org/10.3390/medicina56080373
APA StyleDonegani, M. I., Ferrarazzo, G., Marra, S., Miceli, A., Raffa, S., Bauckneht, M., & Morbelli, S. (2020). Positron Emission Tomography-Based Response to Target and Immunotherapies in Oncology. Medicina, 56(8), 373. https://doi.org/10.3390/medicina56080373








