Cervical-Vaginal Mucin in Fertility Assessment: CA125 as a Predictor of the Fertile Phase of the Normal Menstrual Cycle
Abstract
1. Introduction
2. Methods
2.1. Qvaginal CA125 Assay
2.2. Mean Normalized Day-Specific Qvaginal CA125 and Rate Of Change During Cycle
2.3. Algorithm to Determine Fertile Start Day (FSD) and Calculation of Conception Rates
2.4. Statistical Methods
3. Results
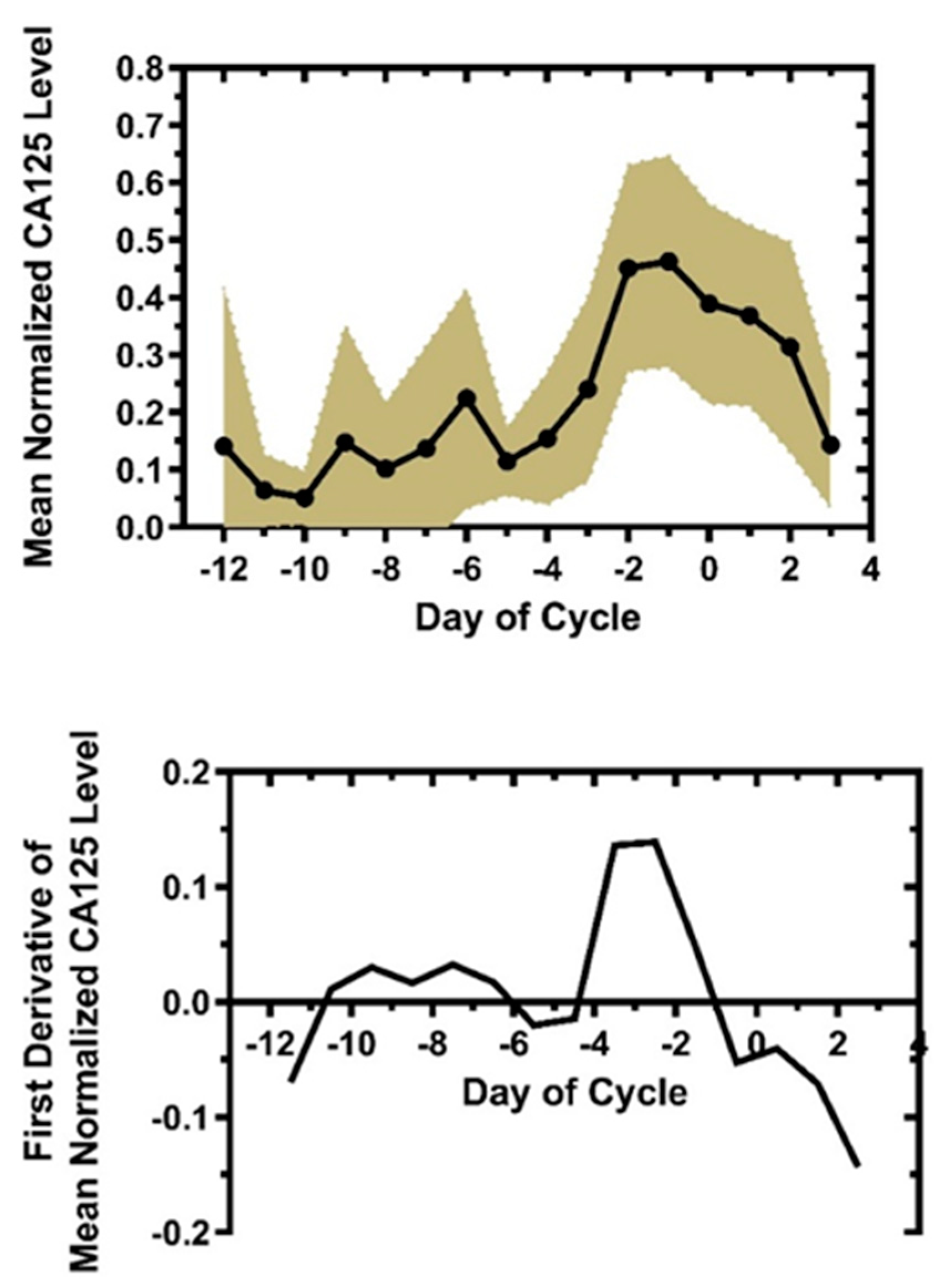
3.1. The Rate of Change Signature for Qvaginal CA125
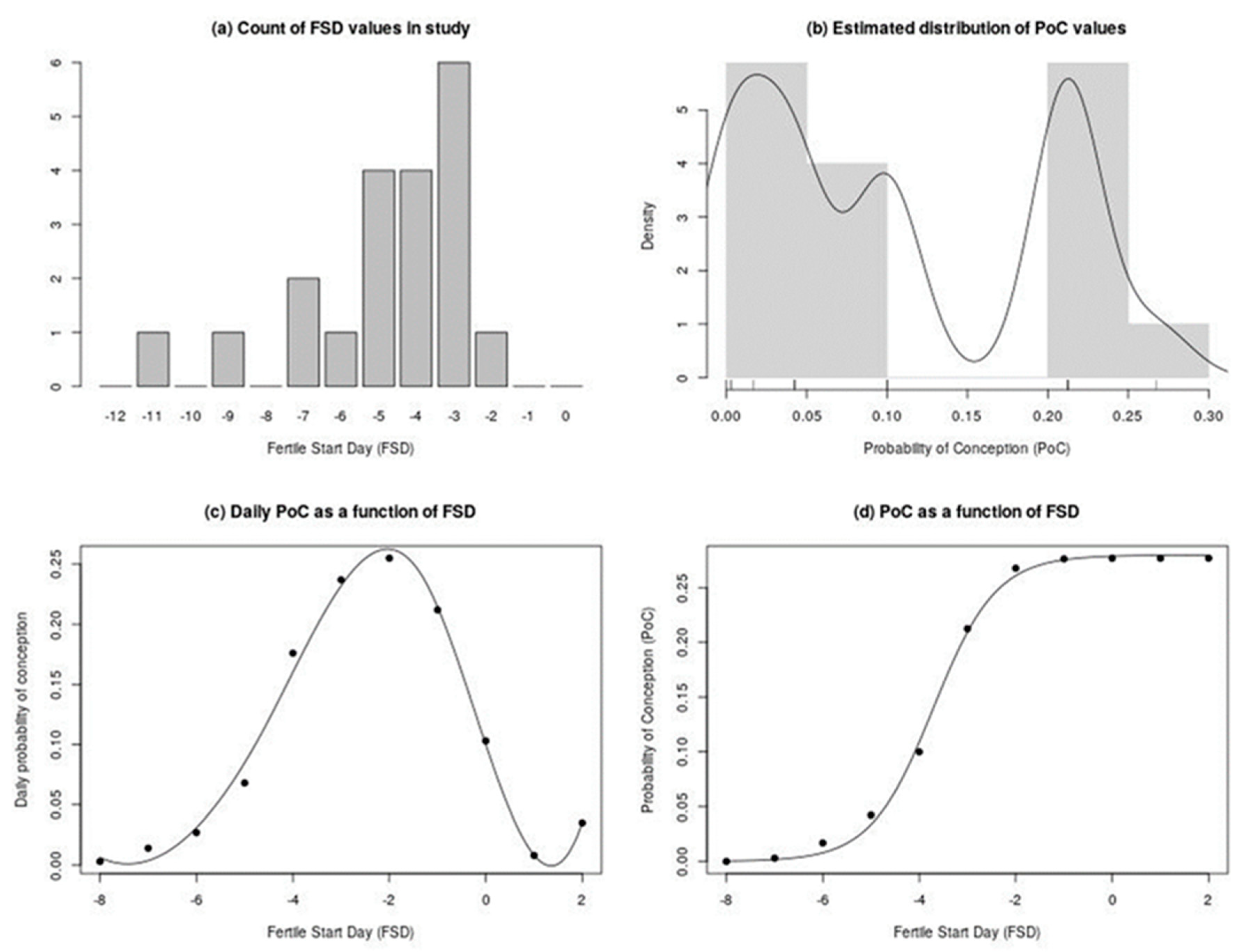
3.2. Fertile Start Day (FSD) for 20 Cycles, 15 Patients, and Estimation of Pregnancy Failure Rates with Qvaginal CA125
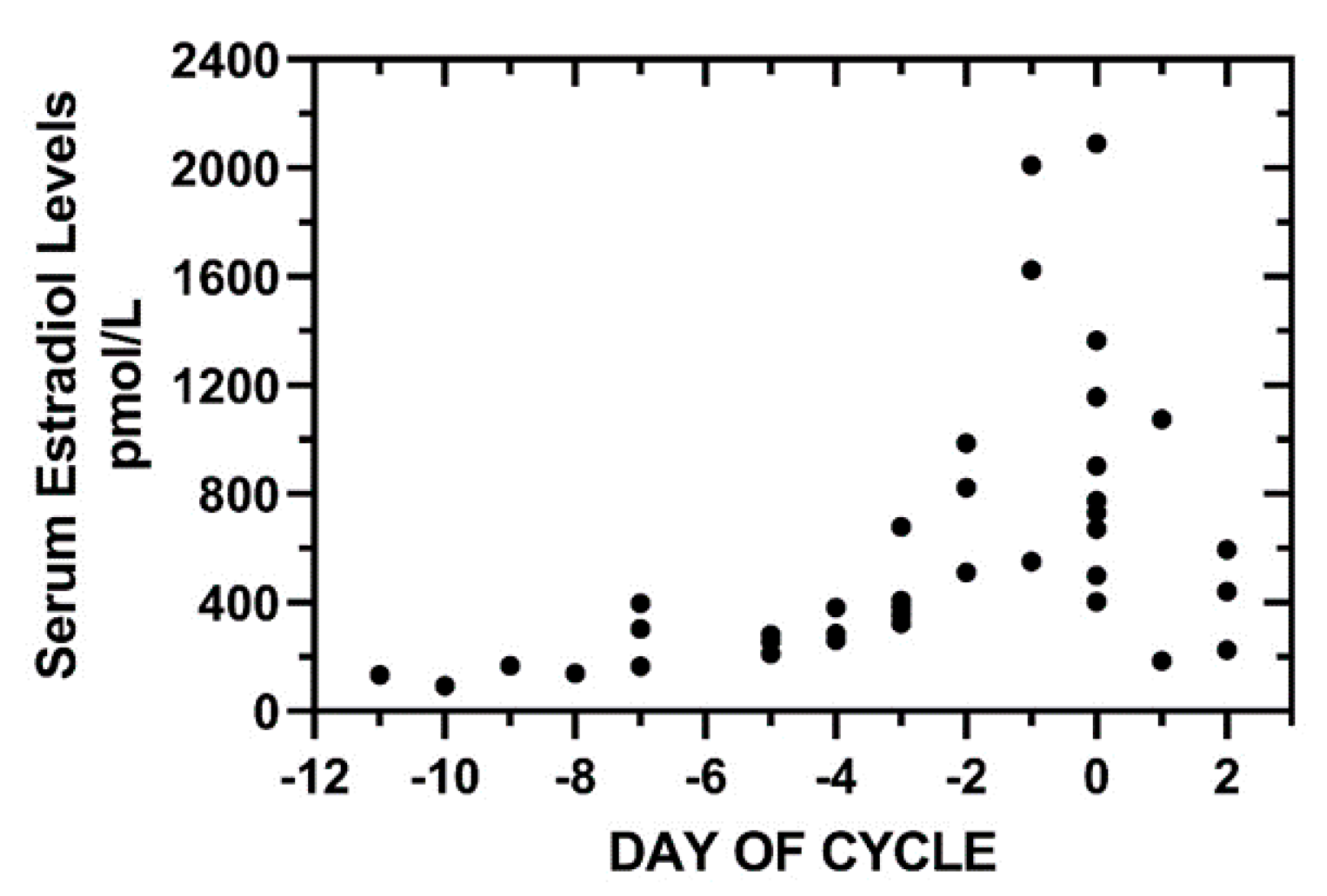
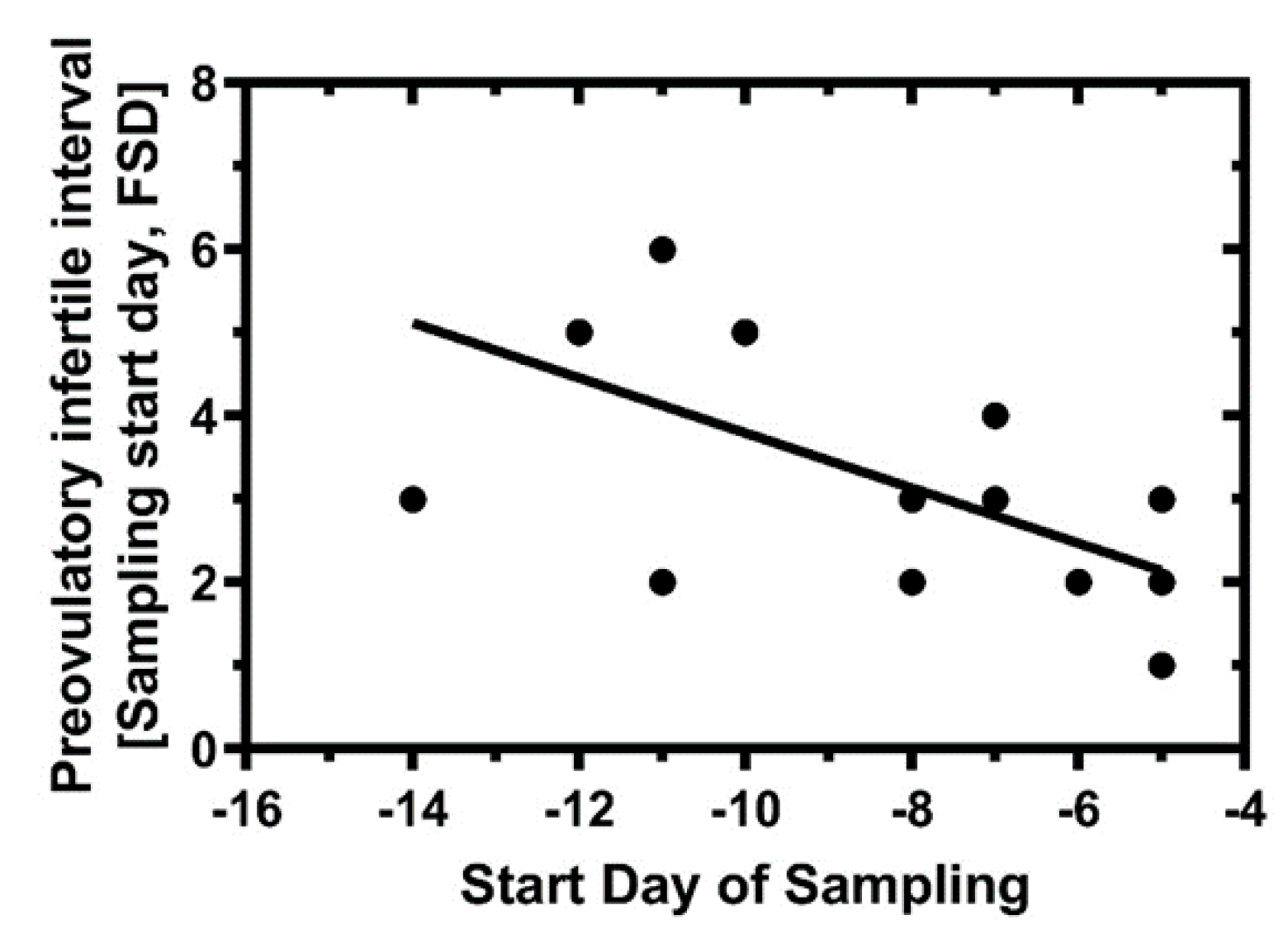
3.3. Preovulatory Estradiol Levels and Predicted Infertility Intervals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fehring, R.J.; Schneider, M.; Barron, M.L. Efficacy of the Marquette Method of natural family planning. MCN Am. J. Matern. Nurs. 2008, 33, 348–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frank-Herrmann, P.; Gnoth, C.; Toledo, E.; Baur, S.; Pyper, C.; Jenetzky, E.; Strowitzki, T.; Freundl, G.; Heil, J. The effectiveness of a fertility awareness based method to avoid pregnancy in relation to a couple’s sexual behaviour during the fertile time: A prospective longitudinal study. Hum. Reprod. 2007, 22, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, T.W.; Stanford, J.B. Creighton Model NaProEducation Technology for avoiding pregnancy. Use effectiveness. J. Reprod. Med. 1998, 43, 495–502. [Google Scholar]
- Bigelow, J.L. Mucus observations in the fertile window: A better predictor of conception than timing of intercourse. Hum. Reprod. 2004, 19, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Usala, S.J.; Usala, F.O.; Haciski, R.; Holt, J.A.; Schumacher, G.F. IgG and IgA content of vaginal fluid during the menstrual cycle. J. Reprod. Med. 1989, 34, 292–294. [Google Scholar] [PubMed]
- Zegels, G.; Van Raemdonck, G.A.; Coen, E.P.; Tjalma, W.A.; Van Ostade, X. Comprehensive proteomic analysis of human cervical-vaginal fluid using colposcopy samples. Proteome Sci. 2009, 7, 17. [Google Scholar] [CrossRef]
- Usala, S.J.; Schumacher, G.F. Volumetric self-sampling of cervicovaginal fluid: A new approach to ovulation timing. Fertil. Steril. 1983, 39, 304–309. [Google Scholar] [CrossRef]
- Wolf, D.P.; Blasco, L.; A Khan, M.; Litt, M. Human cervical mucus. I. Rheologic characteristics. Fertil. Steril. 1977, 28, 41–46. [Google Scholar] [CrossRef]
- Wang, J.; Usala, S.J.; O’brien-Usala, F.; Biggs, W.C.; Vaughn, M.; McKenna, G.B. The Fertile and Infertile Phases of the Menstrual Cycle are Signaled by Cervical-Vaginal Fluid Die Swell Functions. Endocrinology 2009, 19, 291–297. [Google Scholar] [CrossRef]
- Tam, P.Y.; Verdugo, P. Control of mucus hydration as a Donnan equilibrium process. Nature 1981, 292, 340–342. [Google Scholar] [CrossRef]
- Carlstedt, I.; Sheehan, J.K. Structure and macromolecular properties of cervical mucus glycoproteins. Symp. Soc. Exp. Boil. 1989, 43, 289–316. [Google Scholar]
- Pluta, K.; Mcgettigan, P.; Reid, C.J.; Browne, J.; Irwin, J.A.; Tharmalingam, T.; Corfield, A.; Baird, A.W.; Loftus, B.; Evans, A.C.O.; et al. Molecular aspects of mucin biosynthesis and mucus formation in the bovine cervix during the periestrous period. Physiol. Genom. 2012, 44, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Andersch-Björkman, Y.; Thomsson, K.A.; Larsson, J.M.H.; Ekerhovd, E.; Hansson, G.C. Large Scale Identification of Proteins, Mucins, and Their O-Glycosylation in the Endocervical Mucus during the Menstrual Cycle. Mol. Cell. Proteom. 2007, 6, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I. Human endocervical mucus. In New Mechanisms for Tissue-Selective Estrogen-Free Contraception, 1st ed.; Croxatto, H., Schurmann, R., Fuhrmann, U., Schellschmidt, I.B., Eds.; Springer-Verlag: New York, NY, USA, 2007; pp. 219–244. [Google Scholar]
- Kesimer, M.; Makhov, A.M.; Griffith, J.D.; Verdugo, P.; Sheehan, J.K. Unpacking a gel-forming mucin: A view of MUC5B organization after granular release. Am. J. Physiol. Cell. Mol. Physiol. 2009, 298, L15–L22. [Google Scholar] [CrossRef]
- Gipson, I.K. Mucins of the human endocervix. Front. Biosci. 2001, 6, 1245–1255. [Google Scholar] [CrossRef]
- Grande, G.; Milardi, D.; Vincenzoni, F.; Pompa, G.; Biscione, A.; Astorri, A.L.; Fruscella, E.; De Luca, A.; Messana, I.; Castagnola, M.; et al. Proteomic characterization of the qualitative and quantitative differences in cervical mucus composition during the menstrual cycle. Mol. BioSyst. 2015, 11, 1717–1725. [Google Scholar] [CrossRef]
- Martinez, A.R.; Thomas, C.M.; Segers, M.F.; Schoemaker, J.; Eskes, T.K. CA-125 levels in cervical mucus during the menstrual cycle. Fertil. Steril. 1994, 61, 843–849. [Google Scholar] [CrossRef]
- Van Putten, J.P.; Strijbis, K. Transmembrane Mucins: Signaling Receptors at the Intersection of Inflammation and Cancer. J. Innate Immun. 2017, 9, 281–299. [Google Scholar] [CrossRef]
- Button, B.; Cai, L.H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. A Periciliary Brush Promotes the Lung Health by Separating the Mucus Layer from Airway Epithelia. Science 2012, 337, 937–941. [Google Scholar] [CrossRef]
- Usala, S.J.; Biggs, W.C.; O’Brien-Usala, F. Vaginal Swab Levels of CA125 Are Related to Time of Cycle in Ovulatory Women. A Pilot Study. J. Reprod. Med. 2015, 60, 287–293. [Google Scholar]
- Freundl, G.; Godehardt, E.; Kern, P.A.; Frank-Herrmann, P.; Koubenec, H.J.; Gnoth, Ch. Estimated maximum failure rates of cycle monitors using daily conception probabilites in the menstrual cycle. Hum. Reprod. 2003, 18, 2628–2633. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.; Masarotto, G. Daily Fecundability. Demogr. Res. 2000, 3, 1–39. [Google Scholar] [CrossRef]
- Scarpa, B. Probabilistic and statistical models for conception. In Wiley StatsRef, 1st ed.; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J., Eds.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2014; pp. 1–20. [Google Scholar]
- Schwartz, D.; MacDonald, P.D.; Heuchel, V. Fecundability, coital frequency and the viability of ova. Popul. Stud. 1980, 34, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2013; pp. 1–549. [Google Scholar]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Application; Cambridge University Press (CUP): Cambridge, UK, 1997. [Google Scholar]
- Dunson, D.B.; Stanford, J.B. Bayesian Inferences on Predictors of Concept1ion Probabilities. Biometrics 2005, 61, 126–133. [Google Scholar] [CrossRef]
- Scarpa, B.; Dunson, D.B.; Colombo, B. Cervical mucus secretions on the day of intercourse: An accurate marker of highly fertile days. Eur. J. Obstet. Gynecol. Reprod. Boil. 2006, 125, 72–78. [Google Scholar] [CrossRef]
- Ecochard, R.; Leiva, R.; Bouchard, T.; Boehringer, H.; Direito, A.; Mariani, A.; Fehring, R. Use of urinary pregnanediol 3-glucuronide to confirm ovulation. Steroids 2013, 78, 1035–1040. [Google Scholar] [CrossRef]
- Bouchard, T.P.; Genuis, S.J. Personal fertility monitors for contraception. Can. Med. Assoc. J. 2010, 183, 73–76. [Google Scholar] [CrossRef][Green Version]
| Fertile Start Day (FSD) | PoC (n = 20 Cycles) | PoC (n = 19 Cycles, No Outlier) | |
|---|---|---|---|
| Number of Cycles | 20 | 20 | 19 |
| Minimum | −11.0 | - | - |
| Maximum | −2.0 | - | - |
| Mean | −4.8 | 0.107 | 0.098 |
| Standard Error of Mean | 0.5 | 0.020 | 0.019 |
| Lower 95% CI of mean | −5.9 | 0.069 | 0.063 |
| Upper 95% CI of mean | −3.7 | 0.148 | 0.138 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trindade, A.A.; Usala, S.J. Cervical-Vaginal Mucin in Fertility Assessment: CA125 as a Predictor of the Fertile Phase of the Normal Menstrual Cycle. Medicina 2020, 56, 304. https://doi.org/10.3390/medicina56060304
Trindade AA, Usala SJ. Cervical-Vaginal Mucin in Fertility Assessment: CA125 as a Predictor of the Fertile Phase of the Normal Menstrual Cycle. Medicina. 2020; 56(6):304. https://doi.org/10.3390/medicina56060304
Chicago/Turabian StyleTrindade, A. Alexandre, and Stephen J. Usala. 2020. "Cervical-Vaginal Mucin in Fertility Assessment: CA125 as a Predictor of the Fertile Phase of the Normal Menstrual Cycle" Medicina 56, no. 6: 304. https://doi.org/10.3390/medicina56060304
APA StyleTrindade, A. A., & Usala, S. J. (2020). Cervical-Vaginal Mucin in Fertility Assessment: CA125 as a Predictor of the Fertile Phase of the Normal Menstrual Cycle. Medicina, 56(6), 304. https://doi.org/10.3390/medicina56060304






