BRCA1 and BRCA2 Gene Mutations and Lung Cancer Risk: A Meta-Analysis
Abstract
1. Introduction
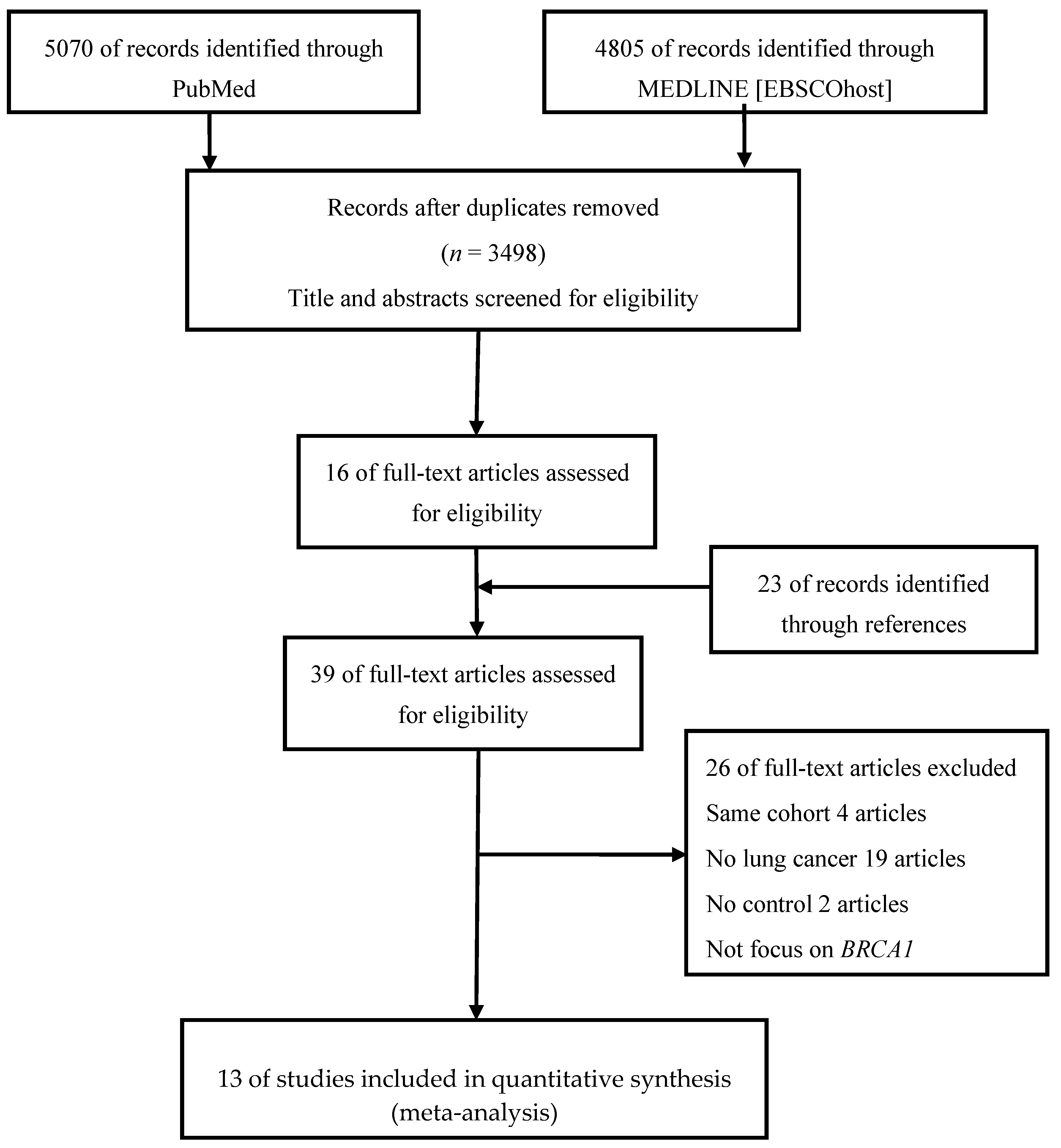
2. Materials and Methods
2.1. Search Strategy and Data Abstraction
2.2. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Walsh, C.S. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol. Oncol. 2015, 137, 343–350. [Google Scholar] [CrossRef]
- Quaas, A.; Heydt, C.; Waldschmidt, D.; Alakus, H.; Zander, T.; Goeser, T.; Kasper, P.; Bruns, C.; Brunn, A.; Roth, W.; et al. Alterations in ERBB2 and BRCA and microsatellite instability as new personalized treatment options in small bowel carcinoma. BMC Gastroenterol. 2019, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Tompa, R. First PARP Inhibitor Ok’d for Breast Cancer. Cancer Discov. 2018, 8, 256–257. [Google Scholar]
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182. [Google Scholar] [CrossRef]
- Dite, G.S.; Whittemore, A.S.; Knight, J.A.; John, E.M.; Milne, R.L.; Andrulis, I.L.; Southey, M.C.; McCredie, M.R.; Giles, G.G.; Miron, A.; et al. Increased cancer risks for relatives of very early-onset breast cancer cases with and without BRCA1 and BRCA2 mutations. Br. J. Cancer 2010, 103, 1103–1108. [Google Scholar] [CrossRef]
- Digennaro, M.; Sambiasi, D.; Tommasi, S.; Pilato, B.; Diotaiuti, S.; Kardhashi, A.; Trojano, G.; Tufaro, A.; Paradiso, A.V. Hereditary and non-hereditary branches of family eligible for BRCA test: Cancers in other sites. Hered. Cancer Clin. Pract. 2017, 15, 7. [Google Scholar] [CrossRef]
- Mersch, J.; Jackson, M.A.; Park, M.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015, 121, 269–275. [Google Scholar] [CrossRef]
- Thompson, D.; Easton, D.F. Cancer Incidence in BRCA1 mutation carriers. J. Natl. Cancer Inst. 2002, 94, 1358–1365. [Google Scholar] [CrossRef]
- Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Streff, H.; Profato, J.; Ye, Y.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancer Incidence in First- and Second-Degree Relatives of BRCA1 and BRCA2 Mutation Carriers. Oncologist 2016, 21, 869–874. [Google Scholar] [CrossRef]
- Struewing, J.P.; Hartge, P.; Wacholder, S.; Baker, S.M.; Berlin, M.; McAdams, M.; Timmerman, M.M.; Brody, L.C.; Tucker, M.A. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N. Engl. J. Med. 1997, 336, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Johannsson, O.; Loman, N.; Moller, T.; Kristoffersson, U.; Borg, A.; Olsson, H. Incidence of malignant tumours in relatives of BRCA1 and BRCA2 germline mutation carriers. Eur. J. Cancer 1999, 35, 1248–1257. [Google Scholar] [CrossRef]
- Van Asperen, C.J.; Brohet, R.M.; Meijers-Heijboer, E.J.; Hoogerbrugge, N.; Verhoef, S.; Vasen, H.F.; Ausems, M.G.; Menko, F.H.; Garcia, E.B.G.; Klijn, J.G.; et al. Netherlands Collaborative Group on Hereditary Breast, Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J. Med. Genet. 2005, 42, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; O’Hara, C.; Khan, S.; Shack, L.; Woodward, E.; Maher, E.R.; Lalloo, F.; Evans, D.G. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam. Cancer 2012, 11, 235–242. [Google Scholar] [CrossRef]
- Kim, H.; Choi, D.H.; Park, W.; Im, Y.H.; Ahn, J.S.; Park, Y.H.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Yu, J.H.; et al. The association between non-breast and ovary cancers and BRCA mutation in first- and second-degree relatives of high-risk breast cancer patients: A large-scale study of Koreans. Hered. Cancer Clin. Pract. 2019, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.; Rosen, B.; Bradley, L.; Kwan, E.; Jack, E.; Vesprini, D.J.; Kuperstein, G.; Abrahamson, J.L.; et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am. J. Hum. Genet. 2001, 68, 700–710. [Google Scholar] [CrossRef]
- Mano, R.; Tamir, S.; Kedar, I.; Benjaminov, O.; Baniel, J.; Tabachnik, T.; Margel, D. Malignant Abnormalities in Male BRCA Mutation Carriers: Results From a Prospectively Screened Cohort. JAMA Oncol. 2018, 4, 872–874. [Google Scholar] [CrossRef]
- Oh, M.; McBride, A.; Yun, S.; Bhattacharjee, S.; Slack, M.; Martin, J.R.; Jeter, J.; Abraham, I. BRCA1 and BRCA2 Gene Mutations and Colorectal Cancer Risk: Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 1178–1189. [Google Scholar] [CrossRef]
- Brose, M.S.; Rebbeck, T.R.; Calzone, K.A.; Stopfer, J.E.; Nathanson, K.L.; Weber, B.L. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J. Natl. Cancer Inst. 2002, 94, 1365–1372. [Google Scholar] [CrossRef]
- Ford, D.; Easton, D.F.; Bishop, D.T.; Narod, S.A.; Goldgar, D.E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994, 343, 692–695. [Google Scholar] [CrossRef]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, S.; de Ruiter, J.R.; Henneman, L.; Brambillasca, C.S.; Lutz, C.; Vaillant, F.; Ferrante, F.; Drenth, A.P.; van der Burg, E.; Siteur, B.; et al. Comparative oncogenomics identifies combinations of driver genes and drug targets in BRCA1-mutated breast cancer. Nat. Commun. 2019, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Alkhushaym, N.; Fallatah, S.; Althagafi, A.; Aljadeed, R.; Alsowaida, Y.; Jeter, J.; Martin, J.R.; Babiker, H.M.; McBride, A.; et al. The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: A meta-analysis. Prostate 2019, 79, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Wu, H.L.; Shi, H.Y.; Su, L.; Zhang, X. The efficacy and safety of olaparib in the treatment of cancers: A meta-analysis of randomized controlled trials. Cancer Manag. Res. 2018, 10, 2553–2562. [Google Scholar] [CrossRef]
- Ko, K.P.; Kim, S.J.; Huzarski, T.; Gronwald, J.; Lubinski, J.; Lynch, H.T.; Armel, S.; Park, S.K.; Karlan, B.; Singer, C.F.; et al. Hereditary Breast Cancer Clinical Study, The association between smoking and cancer incidence in BRCA1 and BRCA2 mutation carriers. Int. J. Cancer 2018, 142, 2263–2272. [Google Scholar] [CrossRef]
- Julian-Reynier, C.; Resseguier, N.; Bouhnik, A.D.; Eisinger, F.; Lasset, C.; Fourme, E.; Nogues, C. Cigarette smoking in women after BRCA1/2 genetic test disclosure: A 5-year follow-up study of the GENEPSO PS cohort. Genet. Med. 2015, 17, 117–124. [Google Scholar] [CrossRef]

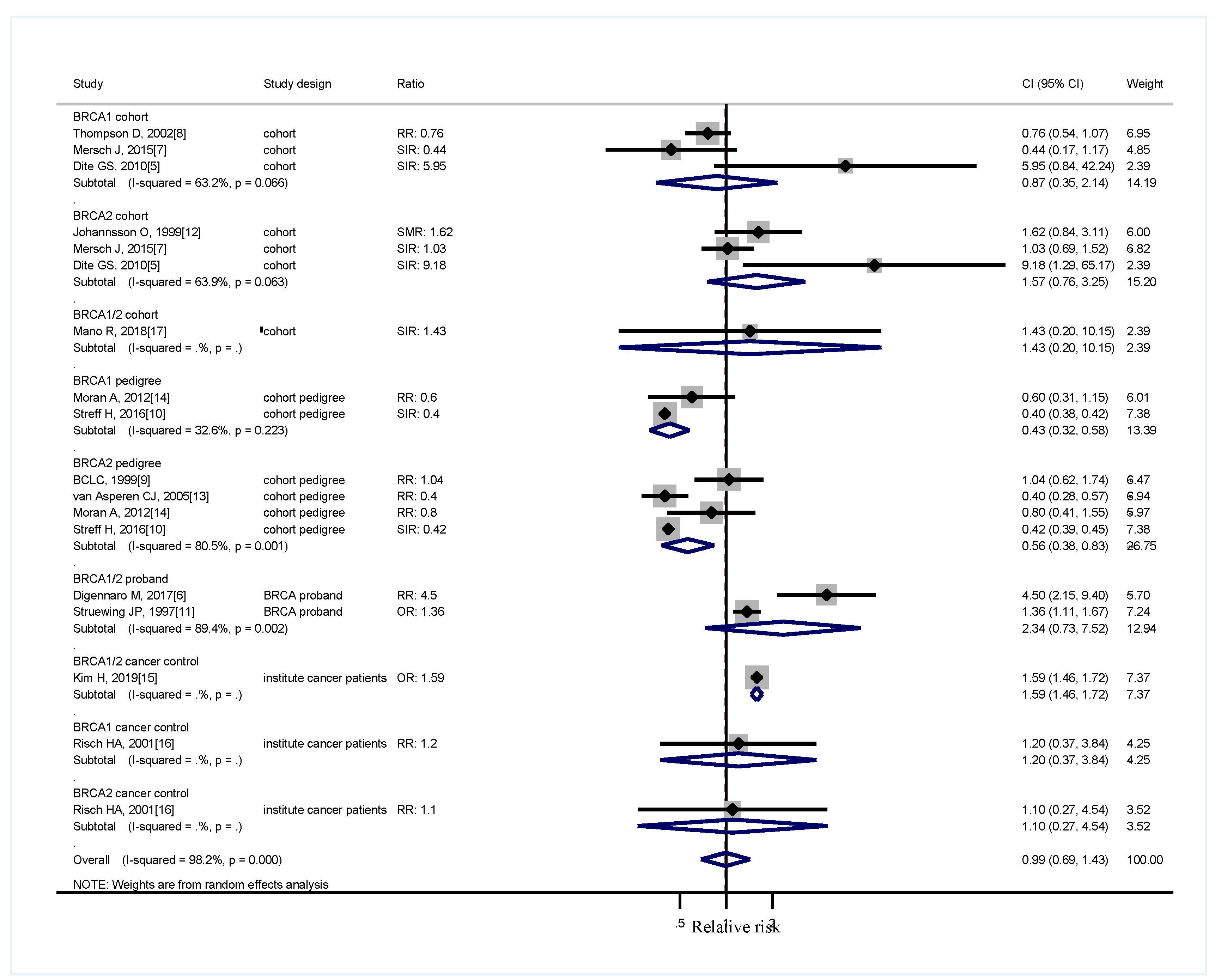

| Cohort Studies | Study Design | Participants BRCA (M,F) * Age ** | Observed Cases + | Controls ++ | Control Cases | Reported Estimated (95% CI) |
|---|---|---|---|---|---|---|
| Cohort studies with ascertained BRCA mutation carriers | ||||||
| Johannsson O et al., 1999 [12] | South Swedish healthcare Cohort | BRCA1 1086 (547,539) BRCA2 684 (366,318) Age (NA) | 6 3 | 2.80 1.85 | Sweden Cancer Registries (1958–1995) | SMR = 2.15 (0.79–4.67) SMR = 1.62 (0.33−4.73) |
| Thompson D et al., 2002 [8] | BCLC (Breast Cancer Linkage Consortium), Western Europe, the US and Canada | BRCA1 2245 (NA) Age by stratification | 5 | 13.05 | Cancer Incidence in Five Continents | RR = 0.76 (0.54–1.07) |
| Dite GS et al., 2010 [5] | Caucasian, SanFrancisco (USA), Ontario (Canada), Melbourne and Sydney (Australia) | BRCA1 25 (NA) BRCA2 17 (NA) Age (NA) | 1 1 | 0.17 0.11 | 1925-1985 Connecticut, USA; 1965–2001 Ontario, Canada; 1983-2001 Australia | SIR = 5.95 (0.84–42.21) SIR = 9.18 (1.29–65.20) |
| Mersch J et al., 2015 [7] | Cohort (MD Anderson) | BRCA1 613 (NA) BRCA2 459 (NA) Mean age 49.3 ± 12.76 | 2 5 | 4.547 4.867 | US Cancer Statistics (1999–2010) | SIR = 0.440 (0.049–1.588) SIR = 1.027 (0.331–2.398) |
| Mano R et al., 2018 [17] | Israel male | BRCA1 117 BRCA2 79 median age 49 | 1 | 0.7 | Age-adjusted cancer incidence, Israeli Jewish male population in Israel-National Cancer register | Not available |
| Cohort studies involving pedigree analysis | ||||||
| BCLC, 1999 [9] | BCLC (Breast Cancer Linkage Consortium), Western Europe, the US and Canada | BRCA2 3728 (NA) Age (NA) | 9 | 11.43 | Cancer Incidence in Five Continents | RR = 1.04 (0.62–1.73) |
| van Asperen CJ et al., 2005 [13] | Cohort, Netherlands | BRCA2 1811 (NA) Ascertain by 50% prior probability of being a carrier Age (NA) | 30 | 40.4 | Eindhoven Cancer Registry to 1990 and Netherlands Cancer Registry from 1990 | RR = 0.4 (0.3–0.6) |
| Moran A et al., 2012 [14] | Cohort United Kingdom | BRCA1 1815 (715,1100) BRCA2 1526 (595,931) Frist degree relatives Age (NA) | 8.2 10.9 | 14.2 13.2 | North West of England (1975–2005) | RR = 0.6 (0.3–1.1) RR = 0.8 (0.4–1.5) |
| Streff H et al., 2016 [10] | Cohort (MD Anderson) | BRCA1 5237 (2401,2836) BRCA2 3795 (1802,1993) First, 2nd degree relatives Age (NA) | 33 30 | 83.8 70.8 | U.S Cancer Statistics (1999–2011) | SIR = 0.40 (0.27–0.55) SIR = 0.42 (0.29–0.61) |
| Cohort studies with special control | ||||||
| Struewing JP et al., 1997 [11] | Cohort, control, branches of the family belonging to the same proband BRCA patients | BRCA 1/2 114 (NA) BRCA(-) 4759 (NA) Age (in categories) | 11 | 337 | Relatives of cases with no mutations | NA |
| Digennaro M et al., 2017 [6] | Cohort, Italy Control, branches of the family belonging to the same proband BRCA patient | BRCA 1/2 1156 (NA) BRCA(-) 1062 (NA) Age mean BRCA1/2 65.9, BRCA (-) 69.1 | 38 | 9 | 2004–2008 consultation in a single center | RR = 4.5 (2.15–9.38) |
| Risch HA et al., 2001 [16] | Cohort, Ontario, Canada; from ovary cancer related family | BRCA1 39 (NA) BRCA2 21 (NA) BRCA(-)455 (NA) Age (in categories) | 4.5% 4.2% | 3.7% | Relatives of cases with no mutations | RR = 1.2 (0.38–3.9) RR = 1.1(0.27–4.6) |
| Kim H et al., 2019 [15] | Cohort, Korea Control, history of cancer other than breast or ovarian cancer | BRCA 1/2 377 (6, 371) BRCA(-) 2178 (13, 2165) Age median 40 | 33 109 | 109 | Breast cancer patients in a single institute | OR = 1.586 (1.057–2.380) |
| Studies | Representativeness of the BRCA Gene | Selection of the Non-BRCA or General Population | Ascertainment of BRCA | Demonstration That Lung Cancer Presented | Study Controls for Initial Age and/or for an Additional Factor | Assessment of Outcome | Was Median Follow-Up 5 Years or More? | Adequacy of Follow-Up (>80%) | Total |
|---|---|---|---|---|---|---|---|---|---|
| Johannsson O [12] | ★ | ★ | ★ | ★ | ★★ | - | - | ★ | 7 |
| Thompson D [8] | ★ | ★ | ★ | ★ | -,- | - | - | - | 4 |
| Dite GS [5] | ★ | ★ | ★ | ★ | ★- | ★ | - | - | 5 |
| Mersch J [7] | ★ | ★ | ★ | ★ | ★★ | ★ | - | - | 7 |
| Mano R [17] | ★ | ★ | ★ | ★ | ★- | ★ | - | - | 6 |
| BCLC [9] | ★ | ★ | ★ | ★ | -,- | - | - | - | 4 |
| van Asperen CJ [13] | ★ | ★ | ★ | ★ | ★★ | - | - | - | 6 |
| Moran A [14] | ★ | ★ | ★ | ★ | ★ ★ | ★ | - | - | 7 |
| Streff H [10] | ★ | ★ | ★ | ★ | ★★ | - | - | - | 6 |
| Digennaro M [6] | ★ | ★ | ★ | ★ | ★★ | - | - | - | 6 |
| Kim H [15] | ★ | ★ | ★ | ★ | -,- | - | - | - | 4 |
| Struewing JP [11] | ★ | ★ | ★ | ★ | -,- | - | - | - | 4 |
| Risch HA [16] | ★ | ★ | ★ | ★ | -,- | - | - | - | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Lee, Y.-C.; Li, C.-Y.; Lee, Y.-L.; Chen, B.-L. BRCA1 and BRCA2 Gene Mutations and Lung Cancer Risk: A Meta-Analysis. Medicina 2020, 56, 212. https://doi.org/10.3390/medicina56050212
Lee Y-C, Lee Y-C, Li C-Y, Lee Y-L, Chen B-L. BRCA1 and BRCA2 Gene Mutations and Lung Cancer Risk: A Meta-Analysis. Medicina. 2020; 56(5):212. https://doi.org/10.3390/medicina56050212
Chicago/Turabian StyleLee, Yen-Chien, Yang-Cheng Lee, Chung-Yi Li, Yen-Ling Lee, and Bae-Ling Chen. 2020. "BRCA1 and BRCA2 Gene Mutations and Lung Cancer Risk: A Meta-Analysis" Medicina 56, no. 5: 212. https://doi.org/10.3390/medicina56050212
APA StyleLee, Y.-C., Lee, Y.-C., Li, C.-Y., Lee, Y.-L., & Chen, B.-L. (2020). BRCA1 and BRCA2 Gene Mutations and Lung Cancer Risk: A Meta-Analysis. Medicina, 56(5), 212. https://doi.org/10.3390/medicina56050212






