Association Between the Cerebral Autoregulation Index (Pressure Reactivity), Patient’s Clinical Outcome, and Quality of ABP(t) and ICP(t) Signals for CA Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Post-Hoc Analysis
2.2. Statistical Analysis
3. Results
3.1. Sensitivity and Specificity
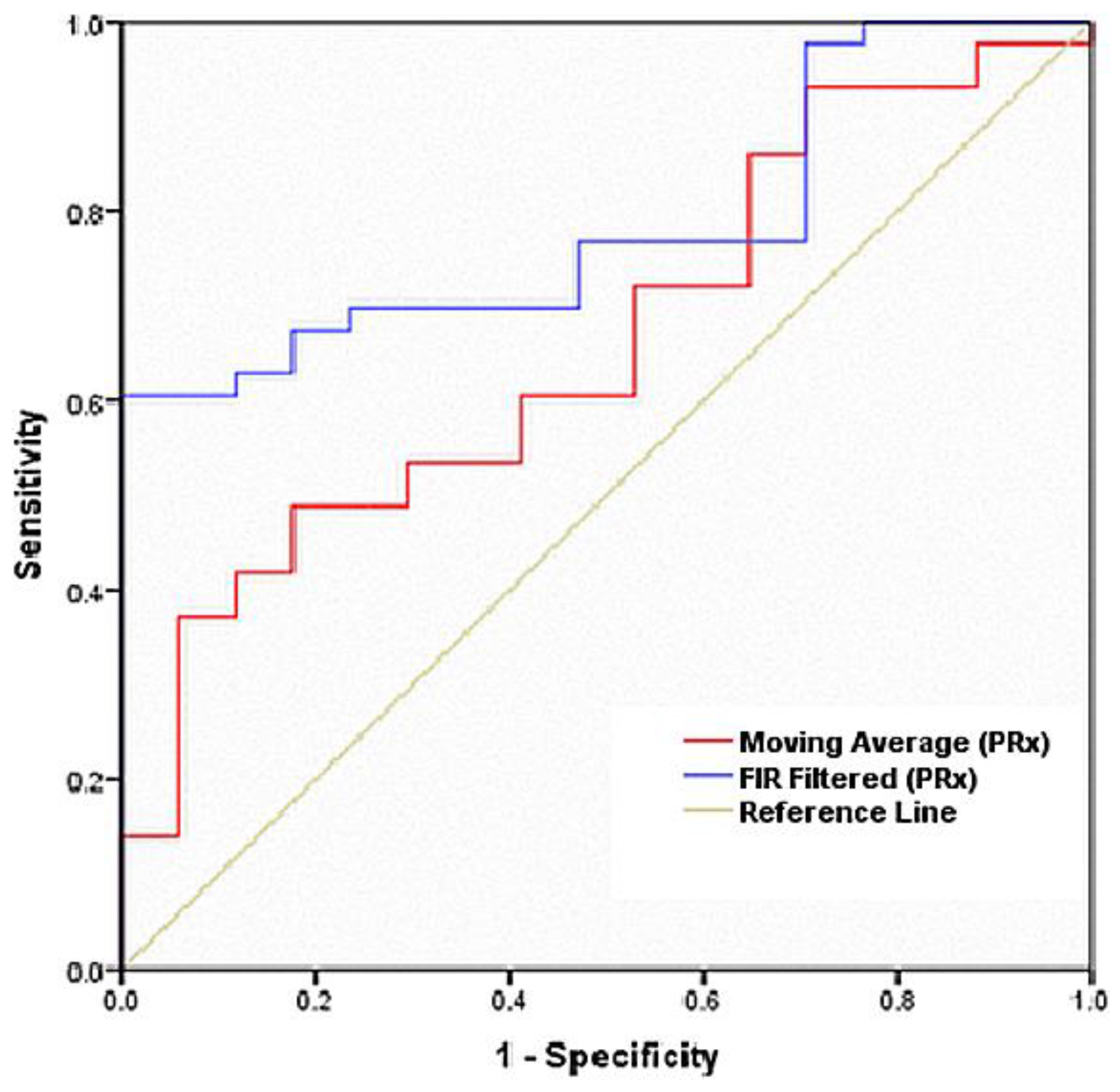
3.2. Receiver Operating Characteristic Curve
3.3. Independent t-test
4. Discussion
5. Conclusions
Limitations of the Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roozenbeek, B.; Maas, A.I.R.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef]
- Stein, S.C.; Georgoff, P.; Meghan, S.; Mizra, K.; Sonnad, S.S. 150 Years of Treating Severe Traumatic Brain Injury: A Systematic Review of Progress in Mortality. J. Neurotrauma 2010, 27, 1343–1353. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K. Traumatic brain injury: Rethinking ideas and approaches. Lancet Neurol. 2012, 11, 12–13. [Google Scholar] [CrossRef]
- Donnelly, J.; Budohoski, K.P.; Smielewski, P.; Czosnyka, M. Regulation of the cerebral circulation: Bedside assessment and clinical implications. Crit. Care 2016, 20, 129. [Google Scholar] [CrossRef]
- Petkus, V.; Preiksaitis, A.; Krakauskaite, S.; Zubaviciute, E.; Rocka, S.; Rastenyte, D.; Vosylius, S.; Ragauskas, A. Benefit on optimal cerebral perfusion pressure targeted treatment for traumatic brain injury patients. J. Crit. Care 2017, 41, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Laing, R.J.; Menon, D.; Pickard, J.D. Continuous assessment of the cerebral vasomotorreactivity in head injury. Neurosurgery 1997, 41, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Smielewski, P.; Lavinio, A.; Czosnyka, Z.; Pickard, J.D. A synopsis of brain pressures: Which? when? Are they all useful? Neurol. Res. 2007, 29, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Maurits, N.M.; Aries, M.J.; Czosnyka, M.; Ercole, A.; Donnelly, J.; Cardim, D.; Kim, D.J.; Dias, C.; Cabeleira, M. Monitoring of Optimal Cerebral Perfusion Pressure in Traumatic Brain Injured Patients Using a Multi-Window Weighting Algorithm. J. Neurotrauma 2017, 34, 3081–3088. [Google Scholar] [CrossRef]
- Kasprowicz, M.; Schmidt, E.; Kim, D.J.; Haubrich, C.; Czosnyka, Z.; Smielewski, P.; Czosnyka, M. Evaluation of the cerebrovascular pressure reactivity index using non-invasive finapres arterial blood pressure. Physiol. Meas. 2010, 31, 1217–1228. [Google Scholar] [CrossRef]
- Petkus, V.; Krakauskaite, S.; Chomskis, R.; Bartusis, L.; Ragauskas, A.; Preiksaitis, A.; Rocka, S. Novel technology of non-invasive cerebrovascular autoregulation monitoring. Proc. Comput. Based Med. Syst. 2014, 427–430. [Google Scholar] [CrossRef]
- Liu, X.; Donnelly, J.; Czosnyka, M.; Aries, M.J.; Brady, K.; Cardim, D.; Robba, C.; Cabeleira, M.; Kim, D.J.; Haubrich, C.; et al. Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: A retrospective study. PLoS Med. 2017, 14, e1002348. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.W.; Kasprowicz, M.; Smielewski, P.; Santos, E.; Pickard, J.; Czosnyka, M. Short pressure reactivity index versus long pressure reactivity index in the management of traumatic brain injury. J. Neurosurg. 2015, 122, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.; Daubaris, G.; Ragaisis, V.; Petkus, V. Implementation of non-invasive brain physiological monitoring concepts. Med. Eng. Phys. 2003, 25, 667–678. [Google Scholar] [CrossRef]
- Ragauskas, A.; Daubaris, G.; Petkus, V.; Raisutis, R. Apparatus and Method of Non-Invasive Cerebrovascular Autoregulation Monitoring. European Patent No. 2111787 B1, 23 March 2011. [Google Scholar]
- Sorrentino, E.; Diedler, J.; Kasprowicz, M.; Budohoski, K.P.; Haubrich, C.; Smielewski, P.; Outtrim, J.G.; Manktelow, A.; Hutchinson, P.J.; Pickard, J.D.; et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit. Care 2012, 16, 258–266. [Google Scholar] [CrossRef]
- Lavinio, A.; Ene-Iordache, B.; Nodari, I.; Girardini, A.; Cagnazzi, E.; Rasulo, F.; Smielewski, P.; Czosnyka, M.; Latronico, N. Cerebrovascular reactivity and autonomic drive following traumatic brain injury. Acta Neurochir. Suppl. 2008, 102, 3–7. [Google Scholar]
- Needham, E.; McFadyen, C.; Newcombe, V.; Synnot, A.J.; Czosnyka, M.; Menon, D. Cerebral Perfusion Pressure Targets Individualized to Pressure-Reactivity Index in Moderate to Severe Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2017, 34, 963–970. [Google Scholar] [CrossRef]
- Steiner, L.A.; Czosnyka, M.; Piechnik, S.K.; Smielewski, P.; Chatfield, D.; Menon, D.K.; Pickard, J.D. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med. 2002, 30, 733–738. [Google Scholar] [CrossRef]
- Aries, M.J.; Czosnyka, M.; Budohoski, K.P.; Steiner, L.A.; Lavinio, A.; Kolias, A.G.; Hutchinson, P.J.; Brady, K.M.; Menon, D.K.; Pickard, J.D.; et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit. Care Med. 2012, 40, 2456–2463. [Google Scholar] [CrossRef]
- Lazaridis, C.; Smielewski, P.; Steiner, L.A.; Brady, K.M.; Hutchinson, P.; Pickard, J.D.; Czosnyka, M. Optimal cerebral perfusion pressure: Are we ready for it? Neurol. Res. 2013, 35, 138–148. [Google Scholar] [CrossRef]
- Akhondi-Asl, A.; Vonberg, F.W.; Au, C.C.; Tasker, R.C. Meaning of Intracranial Pressure-to-Blood Pressure Fisher-Transformed Pearson Correlation-Derived Optimal Cerebral Perfusion Pressure: Testing Empiric Utility in a Mechanistic Model. Crit. Care Med. 2018, 46, e1160–e1166. [Google Scholar] [CrossRef]
- Ellis, T.; McNames, J.; Goldstein, B. Optimal filter design to compute the mean of cardiovascular pressure signals. IEEE Trans. Biomed. Eng. 2008, 55, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Petkus, V.; Krakauskait, S.; Preikšaitis, A.; Ročka, S.; Chomskis, R.; Ragauskas, A. Association between the outcome of traumatic brain injury patients and cerebrovascular autoregulation, cerebral perfusion pressure, age, and injury grades. Medicina 2016, 52, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kumpaitiene, B.; Svagzdiene, M.; Sirvinskas, E.; Adomaitiene, V.; Petkus, V.; Zakelis, R.; Krakauskaite, S.; Chomskis, R.; Ragauskas, A.; Benetis, R. Cerebrovascular autoregulation impairments during cardiac surgery with cardiopulmonary bypasses are related to postoperative cognitive deterioration: Prospective observational study. Minerva Anestesiol. 2019, 85, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Filip, S.-I. A Robust and Scalable Implementation of the Parks-McClellan Algorithm for Designing FIR Filters. ACM Trans. Math. Softw. 2016, 43, 1–24. [Google Scholar] [CrossRef]
- Florkowski, C.M. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: Communicating the performance of diagnostic tests. The Clinical Biochemist. Reviews 2008, 29 (Suppl. 1), S83–S87. [Google Scholar]
- Vetter, T.R.; Schober, P.; Mascha, E.J. Diagnostic testing and decision-making: Beauty is not just in the eye of the beholder. Anesth. Analg. 2018, 127, 1085–1091. [Google Scholar] [CrossRef]
- Šimundić, A.-M. Measures of Diagnostic Accuracy: Basic Definiti. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27683318ons (accessed on 3 October 2019).
- Kumar, R.; Indrayan, A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatrics 2011, 48, 277–287. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21532099 (accessed on 5 October 2019). [CrossRef]
- Zou, K.H.; O’Malley, A.J.; Mauri, L. Receiver-Operating Characteristic Analysis for Evaluating Diagnostic Tests and Predictive Models. Circulation 2007, 115, 654–657. [Google Scholar] [CrossRef]
- Feng, M.; Loy, L.Y.; Zhang, F.; Guan, C. Artifact removal for intracranial pressure monitoring signals: A robust solution with signal decomposition. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Boston, MA, USA, 30 August–3 September 2011; pp. 797–801. [Google Scholar] [CrossRef]
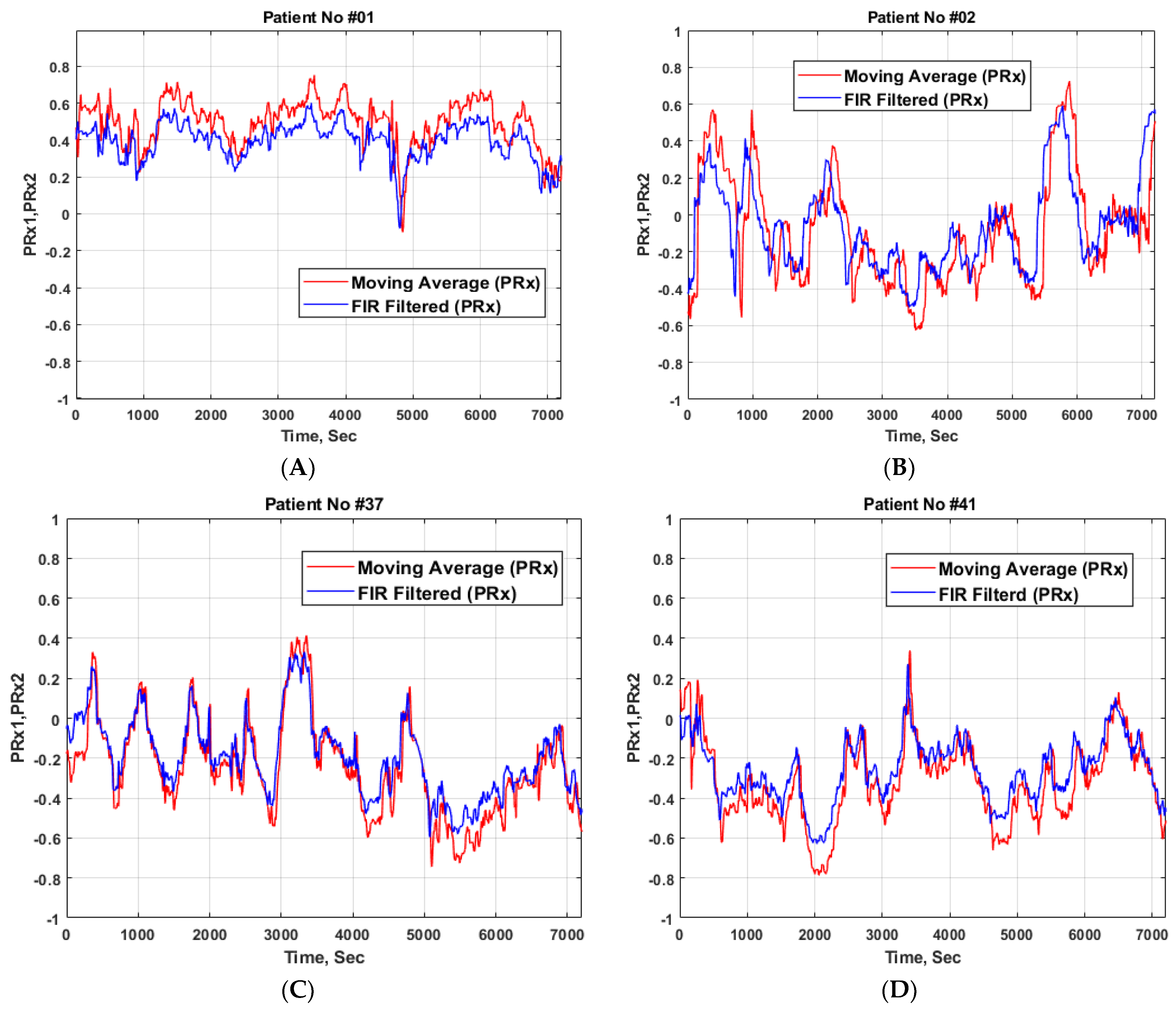
| Survival | Fatal | Total | p-value | |
|---|---|---|---|---|
| Number of patients | 43 | 17 | 60 | - |
| Sex (male/female) | 34/9 | 13/4 | 47/13 | - |
| Age, mean (SD), years | 36.84 (16.09) | 43.25 (11.64) | 38.52 (15.35) | 0.028 |
| GCS, median | 6 (4–7) | 5 (4–6) | 5.50 (5–6) | 0.015 |
| Average PRx1 (moving average filtered), mean (SD) | 0.08 (0.31) | 0.21 (0.34) | 0.11 (0.31) | 0.007 |
| Average PRx2 (optimal filtered), mean (SD) | −0.01(0.36) | 0.26 (0.22) | 0.01(0.37) | 0.001 |
| Outcome | Impaired Autoregulation/Non-survivors (Fatal) | Intact Autoregulation/Survivors |
|---|---|---|
| Positive test (GOS * 1) | (True positive) 12 (M-9, F-3) | (False positive) 8 (M-6, F-2) |
| Negative test (GOS *>1) | (False negative) 5 (M-5, F-0) | (True negative) 35 (M-27, F-8) |
| Outcome | Impaired Autoregulation/ Non-survivors (Fatal) | Intact autoregulation/ Survivors |
|---|---|---|
| Positive Test (GOS * 1) | (True positive) 10 (M-8, F-2) | (False positive) 13 (M-10, F-3) |
| Negative test (GOS *>1) | (False negative) 7 (M-5, F-2) | (True negative) 31 (M-25, F-6) |
| Test Result Variable(s) | Area | Std. Error * | Asymptotic Significance. ** | Asymptotic 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Moving Average (PRx) | 0.661 | 0.075 | 0.054 | 0.515 | 0.807 |
| FIR Filtered (PRx) | 0.785 | 0.058 | 0.001 | 0.671 | 0.900 |
| Test Variable (s) | t | Sig. | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Moving average PRx | 2.123 | 0.054 | 40.319 | 0.040 | 0.29557 | 0.13921 | 0.01428 | 0.57685 |
| FIR PRx | 3.743 | 0.000 | 27.370 | 0.001 | 0.45484 | 0.12151 | 0.20568 | 0.70401 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajpai, B.K.; Preiksaitis, A.; Vosylius, S.; Rocka, S. Association Between the Cerebral Autoregulation Index (Pressure Reactivity), Patient’s Clinical Outcome, and Quality of ABP(t) and ICP(t) Signals for CA Monitoring. Medicina 2020, 56, 143. https://doi.org/10.3390/medicina56030143
Bajpai BK, Preiksaitis A, Vosylius S, Rocka S. Association Between the Cerebral Autoregulation Index (Pressure Reactivity), Patient’s Clinical Outcome, and Quality of ABP(t) and ICP(t) Signals for CA Monitoring. Medicina. 2020; 56(3):143. https://doi.org/10.3390/medicina56030143
Chicago/Turabian StyleBajpai, Basant K., Aidanas Preiksaitis, Saulius Vosylius, and Saulius Rocka. 2020. "Association Between the Cerebral Autoregulation Index (Pressure Reactivity), Patient’s Clinical Outcome, and Quality of ABP(t) and ICP(t) Signals for CA Monitoring" Medicina 56, no. 3: 143. https://doi.org/10.3390/medicina56030143
APA StyleBajpai, B. K., Preiksaitis, A., Vosylius, S., & Rocka, S. (2020). Association Between the Cerebral Autoregulation Index (Pressure Reactivity), Patient’s Clinical Outcome, and Quality of ABP(t) and ICP(t) Signals for CA Monitoring. Medicina, 56(3), 143. https://doi.org/10.3390/medicina56030143





