CPAP Effect on Cardiopulmonary Exercise Testing Performance in Patients with Moderate-Severe OSA and Cardiometabolic Comorbidities
Abstract
1. Introduction
2. Materials and Methods
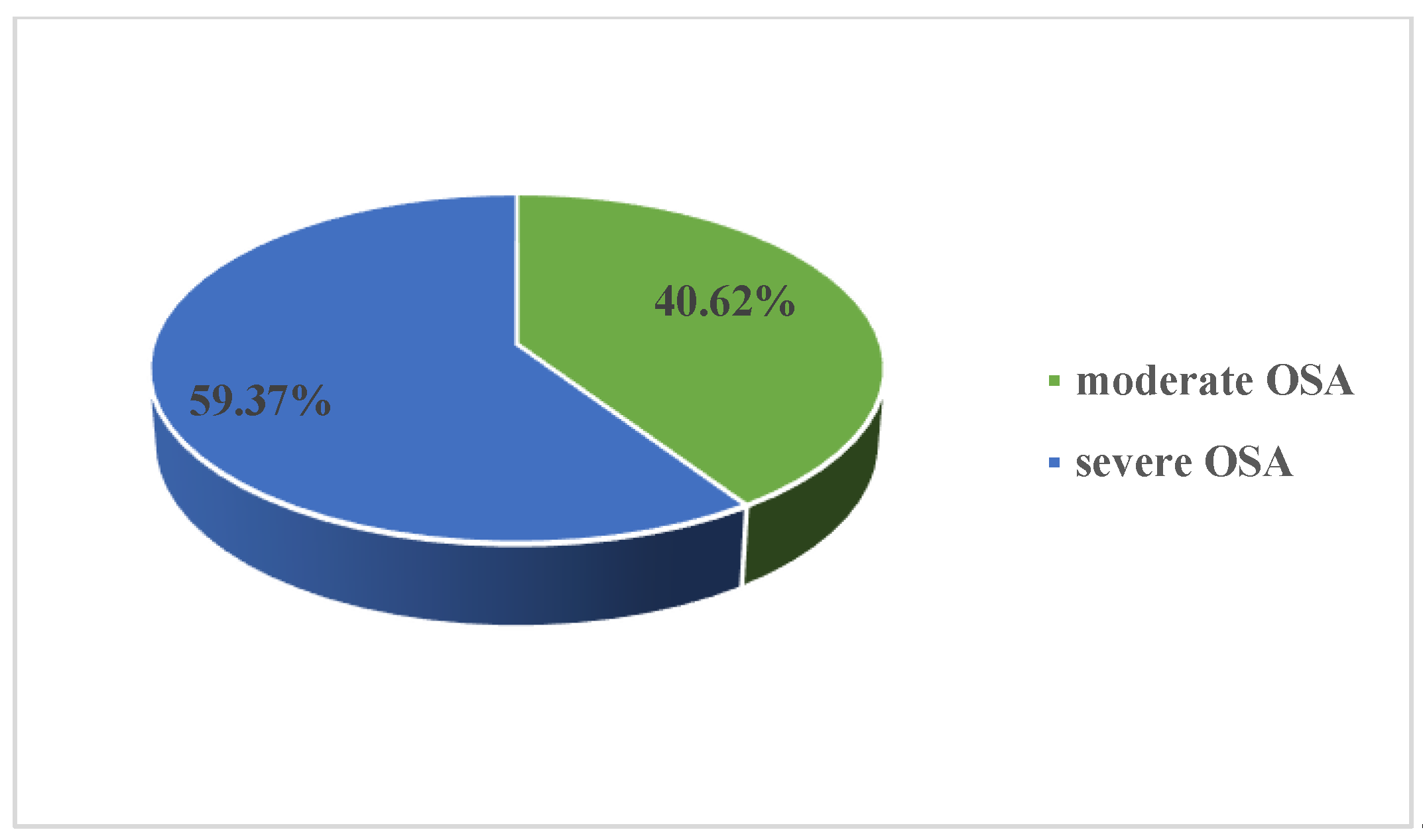
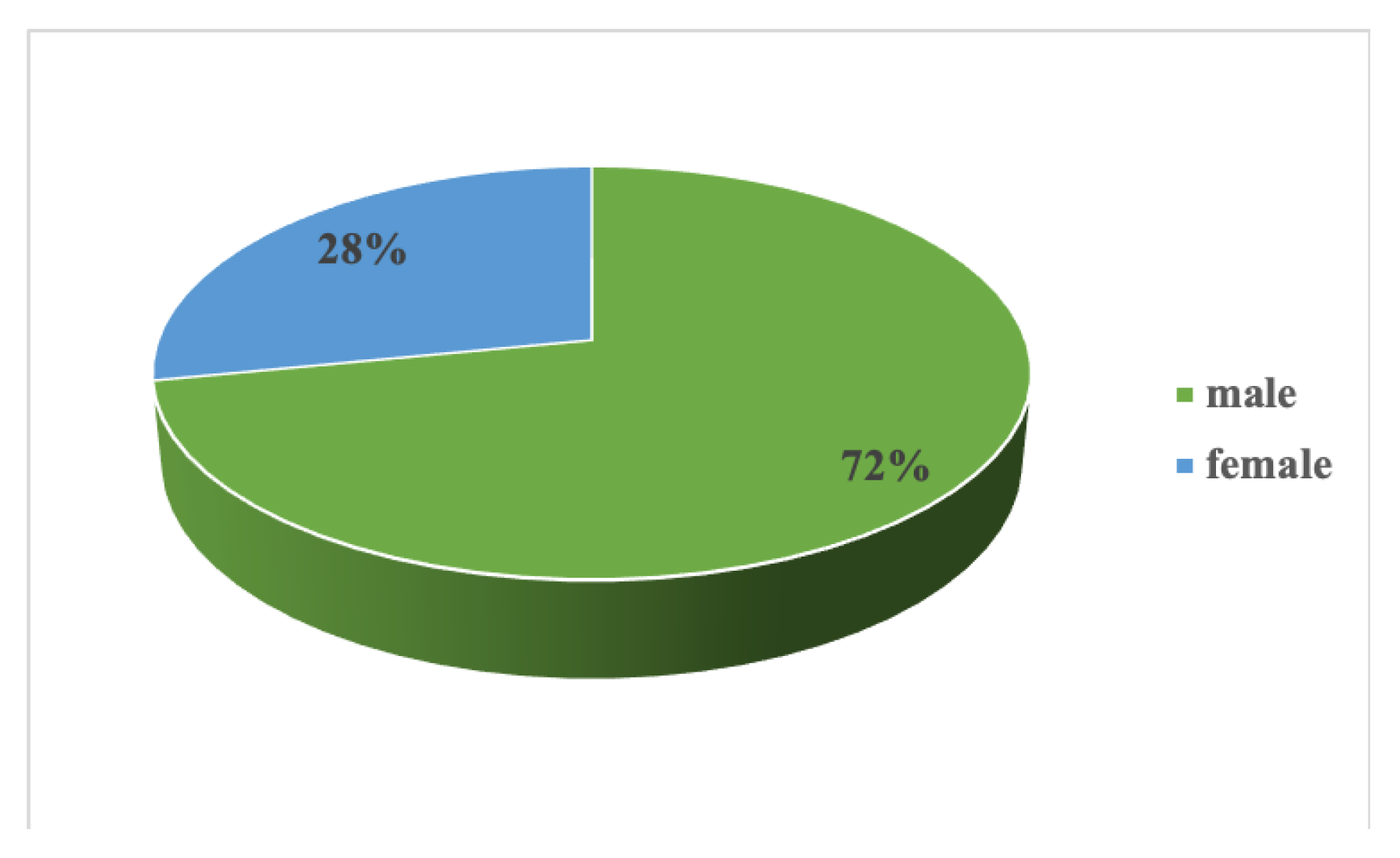
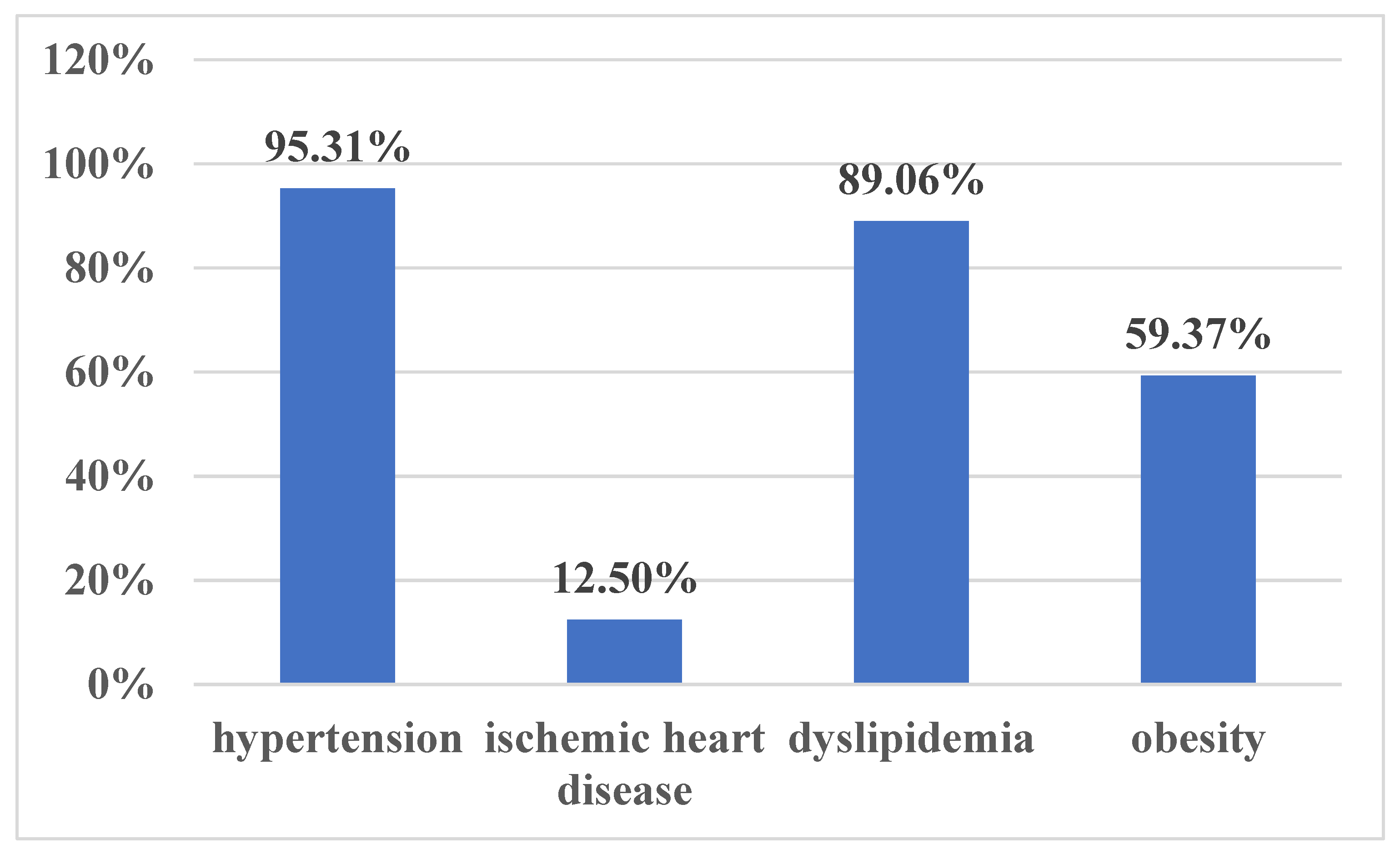
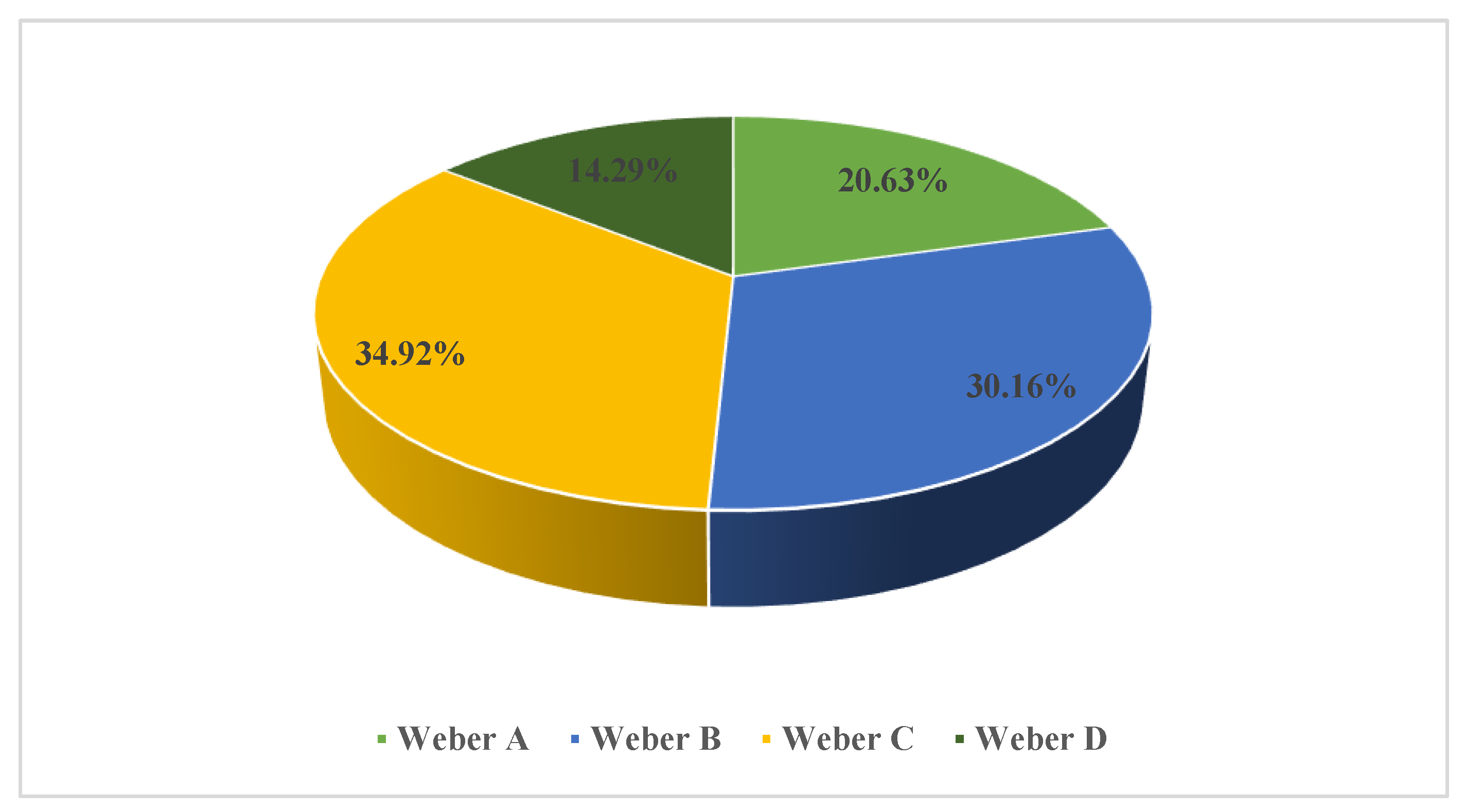
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spicuzza, L.; Caruso, D.; Di Maria, G. Obstructive sleep apnoea syndrome and its management. Ther. Adv. Chronic Dis. 2015, 6, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.; Baril, A.A.; Gagnon, J.F.; Fortin, M.; Décary, A.; Lafond, C.; Desautels, A.; Montplaisir, J.; Gosselin, N. Cognitive impairment in obstructive sleep apnea. Pathol. Biol. (Paris) 2014, 62, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S.; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists). Sleep Apnea and Cardiovascular Disease: Lessons From Recent Trials and Need for Team Science. Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.-L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primer. 2015, 1, 15015. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Fanfulla, F.; Febo, O. Obstructive sleep apnea: One more target in cardiac rehabilitation. Monaldi. Arch. Chest Dis. 2015, 82, 160–164. [Google Scholar] [CrossRef][Green Version]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.-S.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol.-Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hsieh, W.-Y.; Chou, C.-S.; Liaw, S.-F. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir. Physiol. Neurobiol. 2006, 150, 27–34. [Google Scholar] [CrossRef]
- Khan, A.M.; Ashizawa, S.; Hlebowicz, V.; Appel, D.W. Anemia of aging and obstructive sleep apnea. Sleep Breath. 2011, 15, 29–34. [Google Scholar] [CrossRef]
- Stavrou, V.; Bardaka, F.; Karetsi, E.; Daniil, Z.; Gourgoulianis, K.I. Brief Review: Ergospirometry in Patients with Obstructive Sleep Apnea Syndrome. J. Clin. Med. 2018, 7, 191. [Google Scholar] [CrossRef]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Heart Br. Card. Soc. 2007, 93, 1285–1292. [Google Scholar] [CrossRef]
- Berger, M.; Kline, C.E.; Cepeda, F.X.; Rizzi, C.F.; Chapelle, C.; Laporte, S.; Hupin, D.; Raffin, J.; Costes, F.; Hargens, T.A.; et al. Does obstructive sleep apnea affect exercise capacity and the hemodynamic response to exercise? An individual patient data and aggregate meta-analysis. Sleep Med. Rev. 2019, 45, 42–53. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Popovic, R.M.; White, D.P. Upper airway muscle activity in normal women: Influence of hormonal status. J. Appl. Physiol. 1998, 84, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.X.; Jia, S.S.; Liu, Y.H. 17β-Estradiol accentuates contractility of rat genioglossal muscle via regulation of estrogen receptor alpha. Arch Oral. Biol. 2010, 55, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Co, M.; Vanhecke, T.E.; Franklin, B.A.; Sangal, R.B.; Hakmeh, B.; McCullough, P.A. Increased central adiposity in morbidly obese patients with obstructive sleep apnoea. Intern. Med. J. 2011, 41, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Trinder, J.; Kay, A.; Kleiman, J.; Dunai, J. Gender differences in airway resistance during sleep. J Appl. Physiol. 1997, 83, 1986–1997. [Google Scholar] [CrossRef]
- Lin, C.M.; Davidson, T.M.; Ancoli-Israel, S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med. Rev. 2008, 12, 481–496. [Google Scholar] [CrossRef]
- Tapan, Ö.O.; Sevinç, C.; İtil, B.O.; Öztura, İ.; Kayatekin, B.M.; Demiral, Y. Effect of Nasal Continuous Positive Airway Pressure Therapy on the Functional Respiratory Parameters and Cardiopulmonary Exercise Test in Obstructive Sleep Apnea Syndrome. Turk. Thorac. J. 2016, 17, 1–6. [Google Scholar] [CrossRef]
- Przybyłowski, T.; Bielicki, P.; Kumor, M.; Hildebrand, K.; Maskey-Warzechowska, M.; Korczyński, P.; Chazan, R. Exercise capacity in patients with obstructive sleep apnea syndrome. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2007, 58, 563–574. [Google Scholar]
- Cintra, F.D.; Tufik, S.; de Paola, A.; Feres, M.C.; Melo-Fujita, L.; Oliveira, W.; Rizzi, C.; Poyares, D. Cardiovascular profile in patients with obstructive sleep apnea. Arq. Bras. Cardiol. 2011, 96, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kaleth, A.S.; Chittenden, T.W.; Hawkins, B.J.; Hargens, T.A.; Guill, S.G.; Zedalis, D.; Gregg, J.M.; Herbert, W.G. Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med. 2007, 8, 160–168. [Google Scholar] [CrossRef]
- Vanhecke, T.E.; Franklin, B.A.; Zalesin, K.C.; Sangal, R.B.; deJong, A.T.; Agrawal, V.; McCullough, P.A. Cardiorespiratory Fitness and Obstructive Sleep Apnea Syndrome in Morbidly Obese Patients. Chest 2008, 134, 539–545. [Google Scholar] [CrossRef]
- Rizzi, C.F.; Cintra, F.; Mello-Fujita, L.; Rios, L.F.; Mendonca, E.T.; Feres, M.C.; Tufik, S.; Poyares, D. Does obstructive sleep apnea impair the cardiopulmonary response to exercise? Sleep 2013, 36, 547–553. [Google Scholar] [CrossRef]
- Rizzi, C.F.; Cintra, F.; Risso, T.; Pulz, C.; Tufik, S.; de Paola, A.; Poyares, D. Exercise capacity and obstructive sleep apnea in lean subjects. Chest 2010, 137, 109–114. [Google Scholar] [CrossRef]
- Powell, T.A.; Mysliwiec, V.; Aden, J.K.; Morris, M.J. Moderate to Severe Obstructive Sleep Apnea in Military Personnel Is Not Associated With Decreased Exercise Capacity. J. Clin. Sleep Med. JCSM Off. Pub. Am. Acad. Sleep Med. 2019, 15, 823–829. [Google Scholar] [CrossRef]
- Mendelson, M.; Marillier, M.; Bailly, S.; Flore, P.; Borel, J.-C.; Vivodtzev, I.; Doutreleau, S.; Tamisier, R.; Pépin, J.-L.; Verges, S. Maximal exercise capacity in patients with obstructive sleep apnoea syndrome: A systematic review and meta-analysis. Eur. Respir. J. 2018, 51, 1702697. [Google Scholar] [CrossRef]
- Beitler, J.R.; Awad, K.M.; Bakker, J.P.; Edwards, B.A.; DeYoung, P.; Djonlagic, I.; Forman, D.E.; Quan, S.F.; Malhotra, A. Obstructive sleep apnea is associated with impaired exercise capacity: A cross-sectional study. J. Clin. Sleep Med. JCSM Off. Pub. Am. Acad. Sleep Med. 2014, 10, 1199–1204. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, C.-K.; Wu, K.-M.; Chou, C.-S. Effect of treatment by nasal CPAP on cardiopulmonary exercise test in obstructive sleep apnea syndrome. Lung 2004, 182, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Ammann, P.; Münzer, T.; Schoch, O.D.; Korte, W.; Hürny, C.; Myers, J.; Rickli, H. Continuous positive airway pressure improves exercise capacity and heart rate recovery in obstructive sleep apnea. Int. J. Cardiol. 2009, 132, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Quadri, F.; Boni, E.; Pini, L.; Bottone, D.; Venturoli, N.; Corda, L.; Tantucci, C. Exercise tolerance in obstructive sleep apnea-hypopnea (OSAH), before and after CPAP treatment: Effects of autonomic dysfunction improvement. Respir. Physiol. Neurobiol. 2017, 236, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ozsarac, I.; Bayram, N.; Uyar, M.; Kosovali, D.; Gundogdu, N.; Filiz, A. Effects of positive airway pressure therapy on exercise parameters in obstructive sleep apnea. Ann. Saudi Med. 2014, 34, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Kunos, L.; Lazar, Z.; Martinovszky, F.; Tarnoki, A.D.; Tarnoki, D.L.; Kovacs, D.; Forgo, B.; Horvath, P.; Losonczy, G.; Bikov, A. Overnight Changes in Lung Function of Obese Patients with Obstructive Sleep Apnoea. Lung 2017, 195, 127–133. [Google Scholar] [CrossRef]
| Moderate-Severe OSA | Moderate OSA | Severe OSA | p | ||||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | ||
| FVC (L) | 3.76 | 0.92 | 3.84 | 0.85 | 3.71 | 0.97 | 0.46 |
| FVC% (%) | 94.89 | 19.03 | 94.00 | 18.47 | 95.45 | 19.67 | 0.79 |
| FEV1.0 (L) | 3.05 | 0.68 | 3.12 | 0.66 | 3.00 | 0.69 | 0.54 |
| FEV 1.0% (%) | 91.61 | 19.96 | 88.00 | 19.39 | 93.90 | 20.30 | 0.31 |
| FEV1.0/FVC | 77.24 | 2.12 | 77.54 | 1.86 | 77.06 | 2.28 | 0.44 |
| FEV1.0/FVC% | 101.20 | 10.82 | 97.67 | 11.79 | 103.32 | 9.80 | 0.07 |
| PEF (L/sec) | 7.64 | 1.37 | 7.73 | 1.31 | 7.59 | 1.42 | 0.72 |
| PEF% (%) | 77.00 | 19.63 | 75.22 | 18.92 | 78.07 | 20.28 | 0.63 |
| MEF 25 (L/sec) | 1.59 | 0.39 | 1.65 | 0.37 | 1.55 | 0.41 | 0.43 |
| MEF 25% (%) | 69.90 | 23.39 | 61.17 | 24.04 | 75.33 | 21.64 | 0.04 |
| MEF 50 (L/sec) | 4.22 | 0.61 | 4.28 | 0.58 | 4.17 | 0.63 | 0.56 |
| MEF 50% (%) | 75.93 | 25.80 | 68.11 | 27.96 | 80.78 | 23.56 | 0.10 |
| MEF 75 (L/sec) | 6.74 | 1.16 | 6.80 | 1.12 | 6.69 | 1.20 | 0.75 |
| MEF 75% (%) | 76.44 | 20.75 | 72.89 | 21.67 | 78.65 | 20.22 | 0.36 |
| Male | Female | p | |||
|---|---|---|---|---|---|
| Average | SD | Average | SD | ||
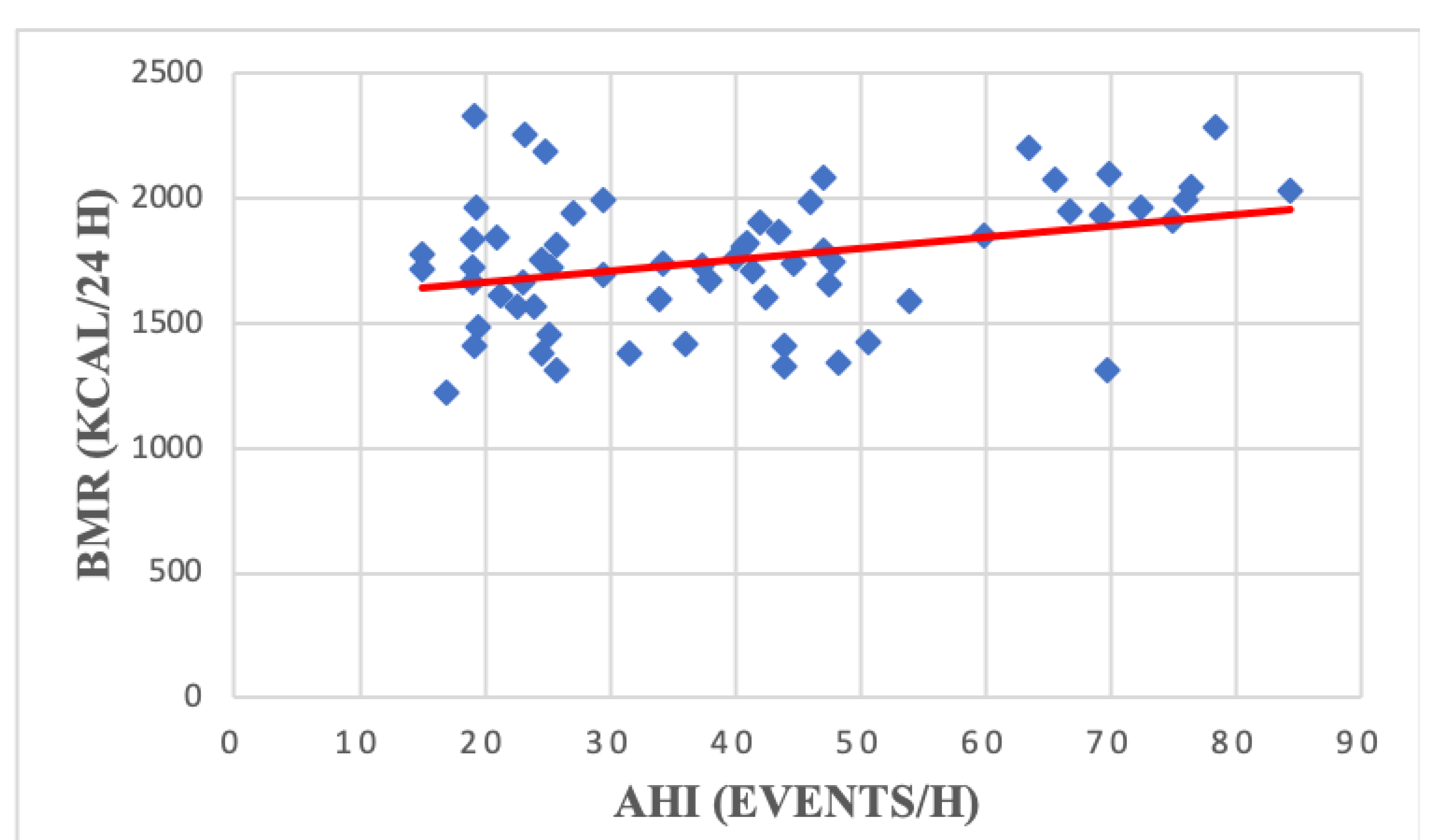
| BMR (kCal/24 h) | 1860.18 | 264.14 | 1494.06 | 187.26 | <0.0000001 |
| Maximal load (W) | 114.85 | 33.54 | 80.78 | 23.41 | 0.0001 |
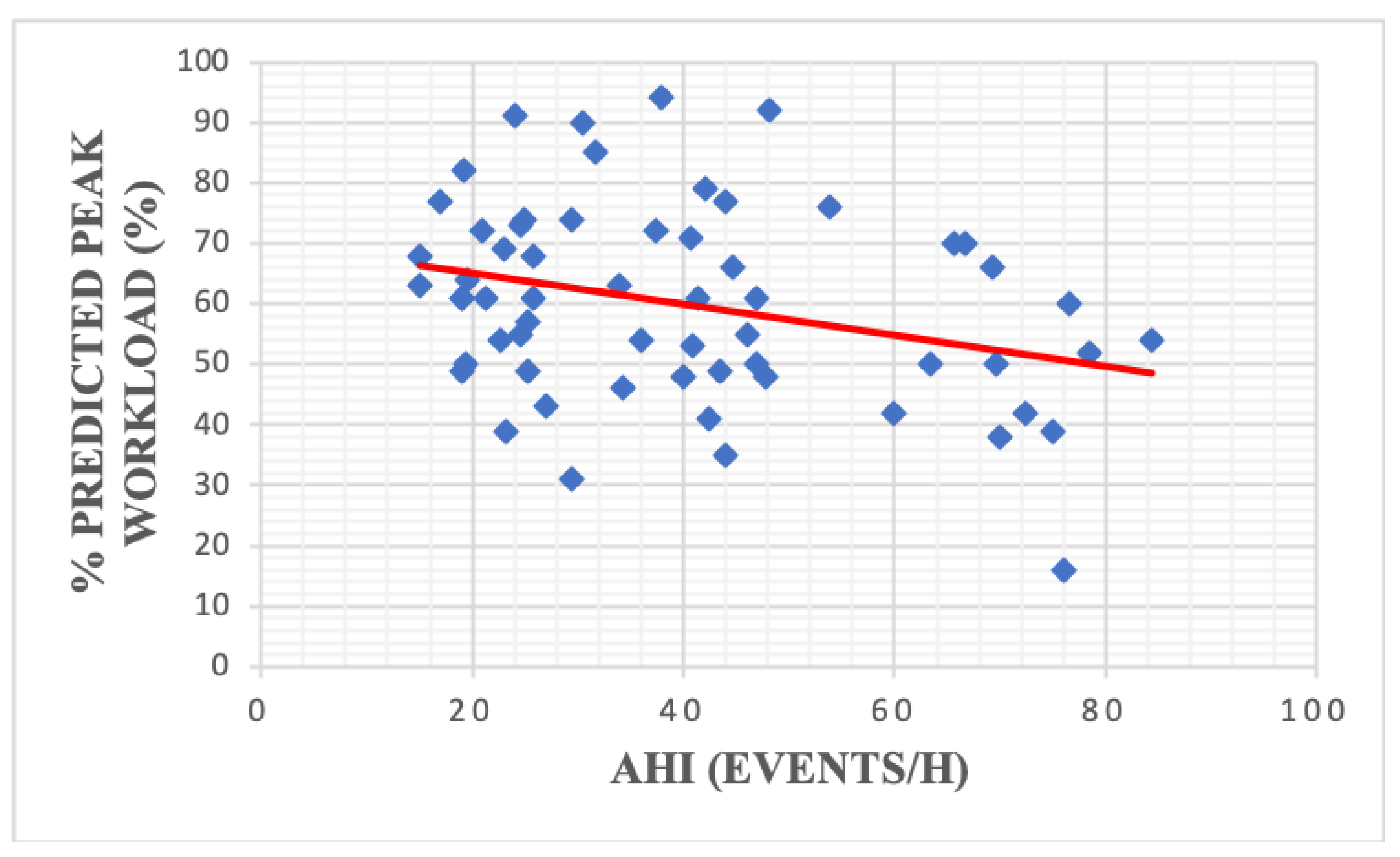
| % predicted maximal load | 56.74 | 16.04 | 70.07 | 15.49 | 0.004 |
| VO2 max | 1553.20 | 504.55 | 1301.67 | 352.11 | 0.07 |
| % predicted VO2 max | 61.59 | 21.73 | 81.83 | 15.56 | 0.0005 |
| AT | 1202.82 | 397.46 | 1083.07 | 288.10 | 0.33 |
| Weight-indexed AT | 11.82 | 3.79 | 11.83 | 3.15 | 0.99 |
| VCO2 max | 1434.52 | 460.30 | 1392.83 | 330.89 | 0.75 |
| VE max (L/min) | 48.64 | 12.88 | 40.29 | 8.79 | 0.02 |
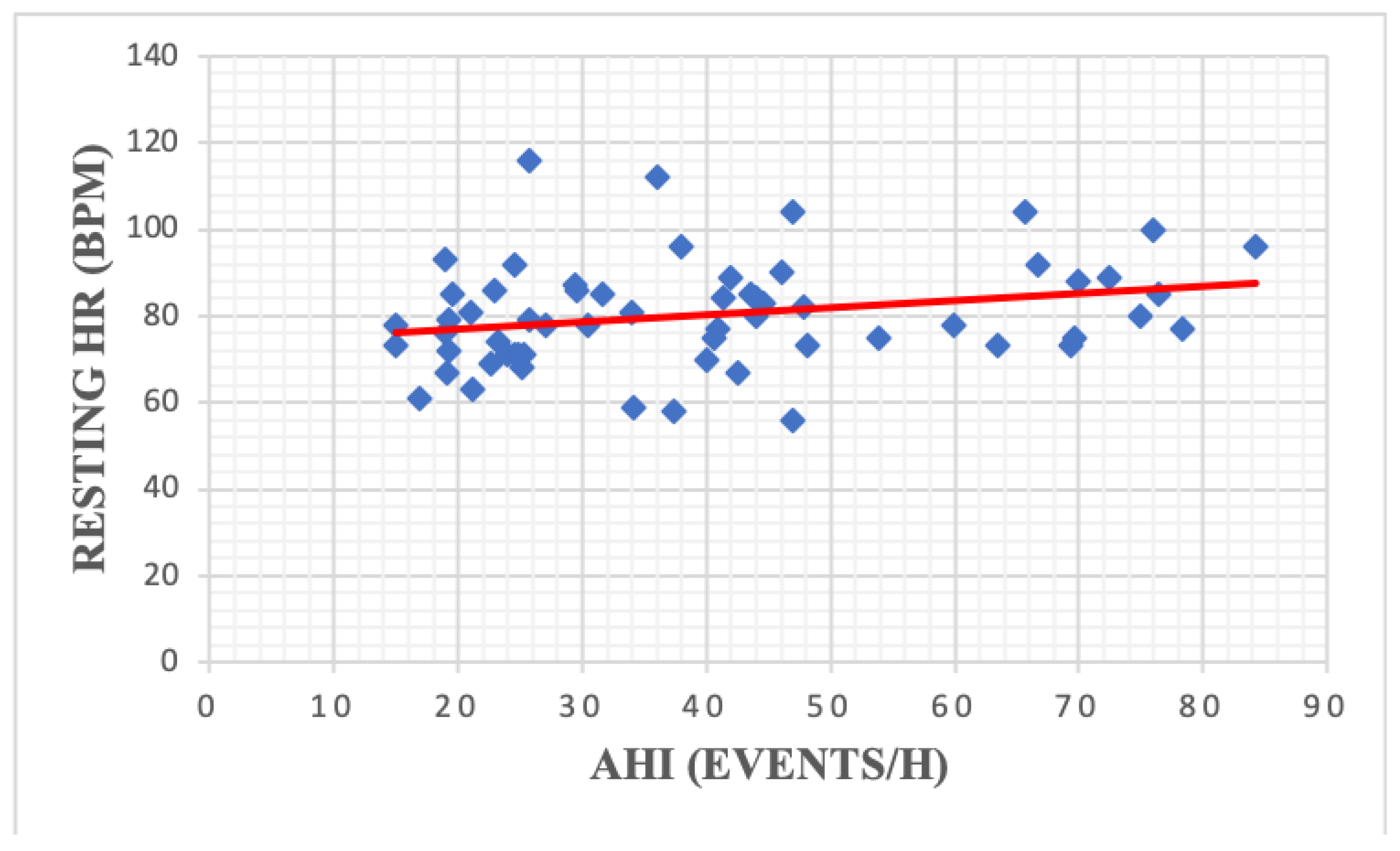
| Resting HR | 79.20 | 12.27 | 83.80 | 15.85 | 0.21 |
| Peak HR | 117.02 | 19.34 | 123.73 | 18.52 | 0.25 |
| % predicted peak HR | 71.48 | 11.62 | 77.80 | 11.29 | 0.07 |
| Peak O2 pulse | 14.46 | 5.46 | 12.37 | 5.40 | 0.20 |
| Weight-indexed O2 pulse | 0.14 | 0.06 | 0.14 | 0.07 | 0.97 |
| Baseline SBP | 124.72 | 16.05 | 127.87 | 16.12 | 0.51 |
| Baseline DBP | 78.46 | 9.83 | 79.27 | 13.29 | 0.78 |
| Peak SBP | 183.16 | 28.01 | 185.33 | 18.85 | 0.80 |
| Peak DBP | 98.64 | 17.61 | 102.53 | 9.52 | 0.46 |
| Moderate-Severe OSA | Moderate OSA | Severe OSA | p | ||||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | ||
| BMR (kCal/24 h) | 1755.57 | 264.14 | 1726.27 | 276.57 | 1776.16 | 256.87 | 0.46 |
| Maximal load (W) | 105.27 | 33.54 | 111.08 | 34.20 | 101.29 | 32.94 | 0.25 |
| % predicted maximal load | 60.02 | 16.04 | 61.84 | 13.81 | 58.75 | 17.50 | 0.46 |
| VO2 max | 1482.45 | 504.55 | 1464.31 | 473.61 | 1494.87 | 530.57 | 0.81 |
| % predicted VO2 max | 67.28 | 21.73 | 67.27 | 21.98 | 67.29 | 21.86 | 0.99 |
| AT | 1168.92 | 397.46 | 1109.32 | 367.95 | 1211.23 | 417.85 | 0.36 |
| Weight-indexed AT | 11.83 | 3.79 | 11.59 | 4.24 | 12.00 | 3.49 | 0.70 |
| VCO2 max | 1422.80 | 460.30 | 1421.54 | 476.78 | 1423.66 | 455.16 | 0.98 |
| VE max (L/min) | 46.58 | 12.88 | 47.12 | 9.21 | 46.21 | 15.03 | 0.78 |
| %VE | 43.05 | 12.51 | 41.89 | 15.86 | 44.05 | 10.40 | 0.57 |
| Resting HR | 80.33 | 12.27 | 77.88 | 11.59 | 82.03 | 12.59 | 0.19 |
| Peak HR | 118.67 | 19.34 | 121.84 | 18.81 | 116.47 | 19.66 | 0.29 |
| % predicted maximum HR | 73.03 | 11.62 | 74.68 | 11.70 | 71.89 | 11.59 | 0.36 |
| Peak O2 pulse | 13.95 | 5.46 | 14.00 | 5.34 | 13.91 | 5.62 | 0.95 |
| Weight-indexed O2 pulse | 0.14 | 0.06 | 0.15 | 0.06 | 0.14 | 0.06 | 0.42 |
| Baseline SBP | 125.49 | 16.05 | 120.04 | 11.64 | 129.28 | 17.69 | 0.02 |
| Baseline DBP | 78.66 | 9.83 | 76.88 | 7.32 | 79.89 | 11.18 | 0.24 |
| Peak SBP | 183.70 | 28.01 | 175.50 | 26.32 | 189.17 | 28.12 | 0.06 |
| Peak DBP | 99.62 | 17.61 | 97.79 | 13.01 | 100.83 | 20.20 | 0.51 |
| r | p | r | p | ||
|---|---|---|---|---|---|
| BMR (kCal/24 h) | 0.33 | 0.008 | VCO2 max | 0.10 | 0.42 |
| Maximal load (W) | −0.07 | 0.55 | VE max (L/min) | 0.05 | 0.72 |
| % predicted maximal load | −0.30 | 0.01 | Resting HR | 0.25 | 0.04 |
| VO2 max | 0.02 | 0.88 | Peak HR | −0.12 | 0.33 |
| % predicted VO2 max | −0.20 | 0.10 | % predicted peak HR | −0.21 | 0.09 |
| AT | 0.15 | 0.28 | Peak O2 pulse | −0.05 | 0.67 |
| Weight-indexed AT | −0.02 | 0.85 | Weight-indexed O2 pulse | −0.19 | 0.13 |
| Baseline | After CPAP | p * | p ** | |||
|---|---|---|---|---|---|---|
| Average | SD | Average | SD | |||
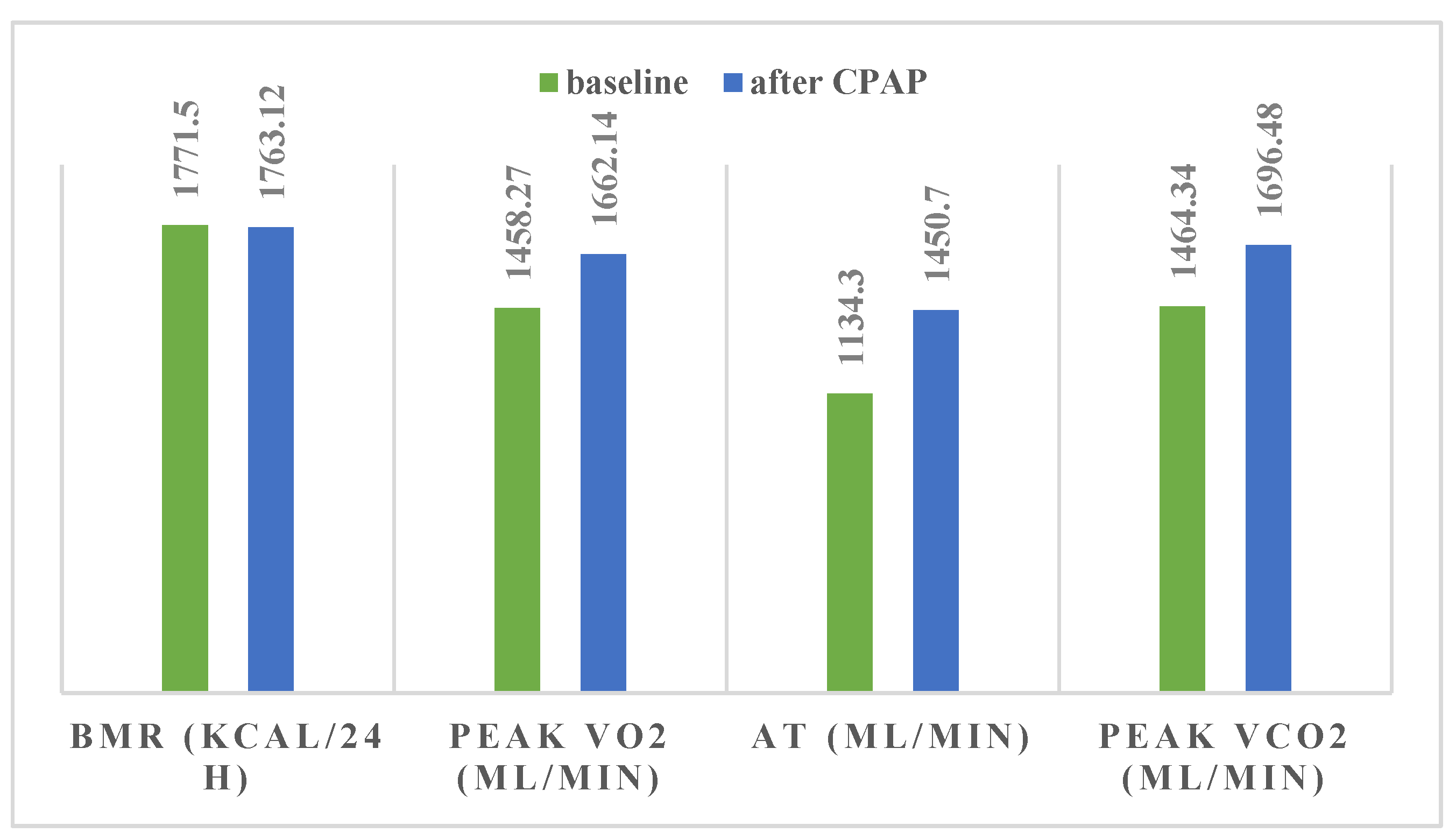
| BMR (kCal/24 h) | 1771.50 | 281.49 | 1763.12 | 273.79 | 0.04 | 0.78 |
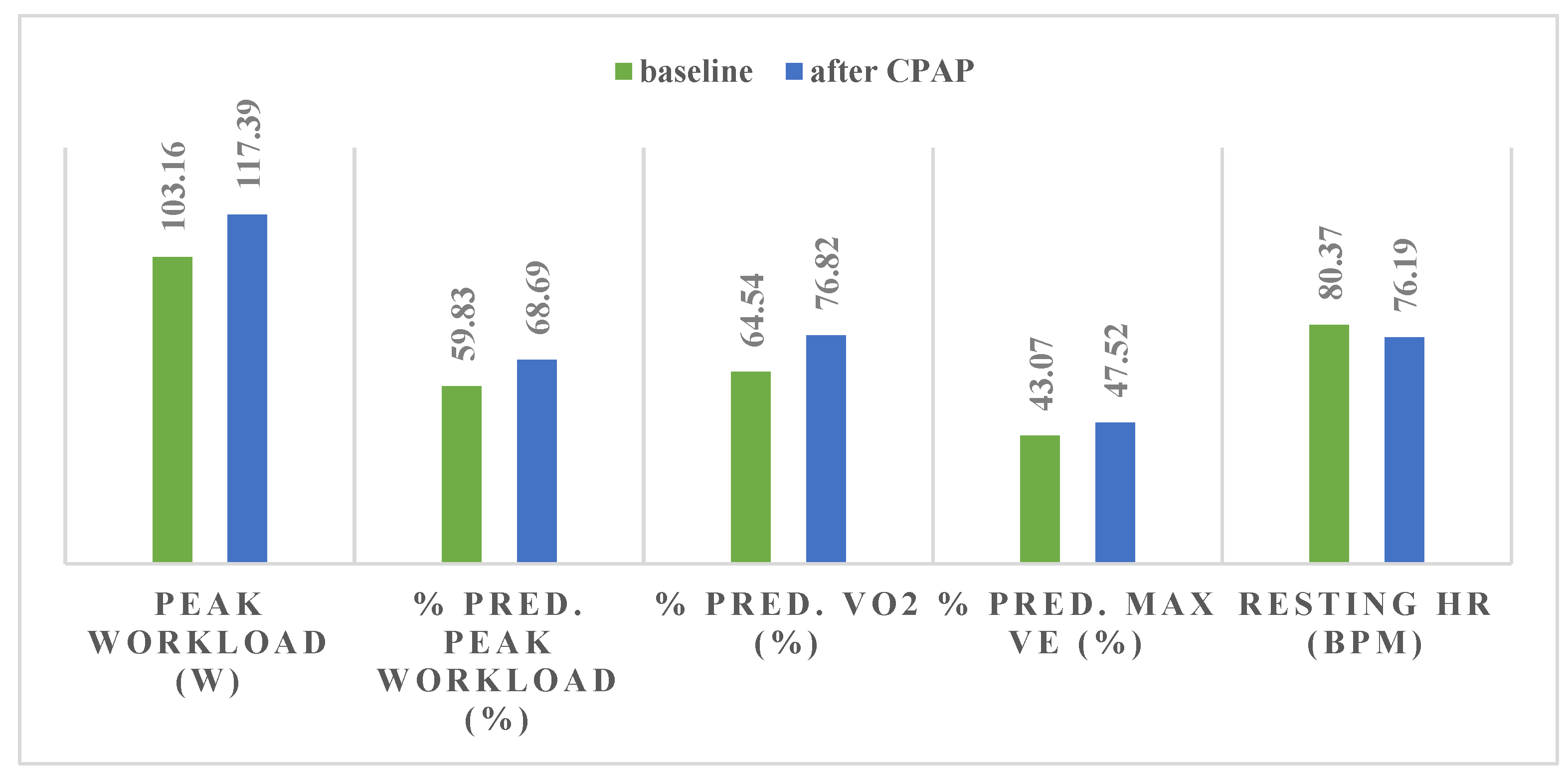
| Maximal load (W) | 103.16 | 34.21 | 117.39 | 36.17 | 0.0004 | 0.04 |
| % predicted maximal load | 59.83 | 16.48 | 68.69 | 14.35 | 0.0001 | 0.01 |
| VO2 max | 1458.27 | 435.29 | 1662.14 | 454.50 | 0.004 | 0.02 |
| % predicted VO2 max | 64.54 | 17.49 | 76.82 | 18.47 | 0.000005 | 0.001 |
| AT | 1134.30 | 419.42 | 1450.70 | 450.54 | 0.001 | 0.08 |
| Weight-indexed AT | 11.41 | 4.07 | 14.62 | 4.66 | 0.001 | 0.07 |
| VCO2 max | 1464.34 | 383.13 | 1696.48 | 465.62 | 0.0006 | 0.01 |
| VE max (L/min) | 46.46 | 13.57 | 51.56 | 14.23 | 0.016 | 0.09 |
| %VE | 43.07 | 12.69 | 47.52 | 11.48 | 0.04 | 0.04 |
| Resting HR | 80.37 | 13.15 | 76.19 | 14.46 | 0.05 | - |
| Peak HR | 118.22 | 20.06 | 121.67 | 23.94 | 0.28 | - |
| % predicted maximum HR | 72.59 | 11.84 | 73.84 | 11.96 | 0.42 | - |
| Peak O2 pulse | 13.59 | 4.14 | 16.05 | 5.83 | 0.007 | 0.02 |
| Weight-indexed O2 pulse | 0.14 | 0.05 | 0.16 | 0.06 | 0.01 | 0.26 |
| Baseline SBP | 126.77 | 17.63 | 122.19 | 15.93 | 0.13 | - |
| Baseline DBP | 78.94 | 10.29 | 77.42 | 8.77 | 0.35 | - |
| Peak SBP | 184.62 | 29.35 | 185.35 | 23.27 | 0.85 | - |
| Peak DBP | 101.52 | 13.74 | 98.33 | 10.40 | 0.11 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zota, I.M.; Stătescu, C.; Sascău, R.A.; Roca, M.; Gavril, R.S.; Vasilcu, T.F.; Boișteanu, D.; Maștaleru, A.; Jitaru, A.; Leon Constantin, M.M.; et al. CPAP Effect on Cardiopulmonary Exercise Testing Performance in Patients with Moderate-Severe OSA and Cardiometabolic Comorbidities. Medicina 2020, 56, 80. https://doi.org/10.3390/medicina56020080
Zota IM, Stătescu C, Sascău RA, Roca M, Gavril RS, Vasilcu TF, Boișteanu D, Maștaleru A, Jitaru A, Leon Constantin MM, et al. CPAP Effect on Cardiopulmonary Exercise Testing Performance in Patients with Moderate-Severe OSA and Cardiometabolic Comorbidities. Medicina. 2020; 56(2):80. https://doi.org/10.3390/medicina56020080
Chicago/Turabian StyleZota, Ioana Mădălina, Cristian Stătescu, Radu Andy Sascău, Mihai Roca, Radu Sebastian Gavril, Teodor Flaviu Vasilcu, Daniela Boișteanu, Alexandra Maștaleru, Alexandra Jitaru, Maria Magdalena Leon Constantin, and et al. 2020. "CPAP Effect on Cardiopulmonary Exercise Testing Performance in Patients with Moderate-Severe OSA and Cardiometabolic Comorbidities" Medicina 56, no. 2: 80. https://doi.org/10.3390/medicina56020080
APA StyleZota, I. M., Stătescu, C., Sascău, R. A., Roca, M., Gavril, R. S., Vasilcu, T. F., Boișteanu, D., Maștaleru, A., Jitaru, A., Leon Constantin, M. M., & Mitu, F. (2020). CPAP Effect on Cardiopulmonary Exercise Testing Performance in Patients with Moderate-Severe OSA and Cardiometabolic Comorbidities. Medicina, 56(2), 80. https://doi.org/10.3390/medicina56020080







