Abstract
Background and Objectives: The aim of this retrospective cohort study was to develop a new score (RA-CHADSV) (rheumatoid arthritis - congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, and vascular disease), modified from the CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, stroke/transient ischemic attack (doubled), vascular disease, age 65–74 years, and female), in predicting the risk of ischemic stroke in rheumatoid arthritis (RA) patients without atrial fibrillation (AF). Materials and Methods: Using the Taiwan’s National Health Insurance Research Database, 592 patients with RA diagnosed between 2000 and 2002 were identified and followed until first occurrence of ischemic stroke or the last available date in the database. Incidence rate ratios (IRR) of ischemic stroke for the CHA2DS2-VASc score were calculated using Poisson regression models. A new prediction score RA-CHADSV was developed using multiple logistic regression analysis with bootstrap validation. Results: The area under the receiver operating characteristic curve of the newly developed RA-CHADSV score and the CHA2DS2-VASc score were 0.73 (95% confidence interval (CI) 0.64–0.82) and 0.70 (95% CI 0.61–0.79), respectively. The RA-CHADSV score was significantly associated with a higher ischemic stroke incidence in the patients who scored ≥1 (adjusted IRR 7.39, p < 0.001). Conclusions: A simplified RA-CHADSV score, with comparable efficiency as the CHA2DS2-VASc score, but easier to use clinically was developed for predicting the risk of ischemic stroke among non-AF RA patients.
1. Introduction
Rheumatoid arthritis (RA) is one of the most prevalent chronic inflammatory polyarthritis with a prevalence of 0.5–1% with female predominance in industrialized countries [1]. The risk of stroke is higher in patients with RA compared with the general population or patients with noninflammatory arthropathies [2,3,4,5]. Patients with RA are also at a higher risk for long-term disability after stroke [6]. Previous research has shown that the presence of autoantibodies, autoantigens, and pro-inflammatory cytokines in autoimmune diseases could accelerate the process of atherosclerosis [7,8]. Abnormal endothelial function and arterial intima-media thickening could cause the formation of atherosclerotic lesions [7], and these lesions are prone to rupture in patients with RA [8,9]. Therefore, patients with RA were associated with a higher risk of stroke [10,11], which might lead to long-term disability after stroke [6] and increased mortality [12].
Several numerical tools have recently been developed for the prediction of ischemic stroke [13,14]. The CHADS2 score (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and stroke [doubled]) and its updated version CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, stroke/transient ischemic attack (doubled), vascular disease, age 65–74 years, and female) are commonly used clinical prediction rules for estimating the risk of major adverse cardiovascular events (MACE) among patients with atrial fibrillation (AF) [15,16,17,18]. These scores are also widely used in the decision making of atrial fibrillation ablation and non-valvular atrial fibrillation (NVAF) to predict the risk of MACE and stroke [19,20]. Recently, the CHA2DS2-VASc has been used for the evaluation of the risk of stroke and MACE in specific populations without AF [21,22], including heart failure, systemic lupus erythematosus, diabetes, hemodialysis, and RA [23,24,25,26]. We proposed that the risk of ischemic stroke might be different in patients with RA. The aim of this secondary retrospective cohort study was to develop a new and simplified score, based on the CHADS2 and CHA2DS2-VASc scores, for predicting the risk of ischemic stroke in RA patients without AF.
2. Materials and Methods
2.1. Data Source
This retrospective cohort study used data from the Longitudinal Health Insurance Database 2000 (LHID 2000). The LHID 2000 contains one million randomly selected patients, excluding medical personnel, from the year 2000 Registry of Beneficiaries of the Taiwan’s National Health Insurance Research Database. No statistical significant differences existed in the age, sex, or health-care costs between the LHID 2000 and all enrollees of the Taiwan’s National Health Insurance (NHI) program. The NHI program is a single-payer universal health insurance plan covering the health care services for over 99% of Taiwan’s residents [27]. The study protocol was approved by the Institutional Review Board of Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan (No. B10004021) on 20 January 2012.
2.2. Study Population
In this study, patients with RA were identified from the LHID 2000 using the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) code 714.0, from 1 January 2000 to 31 December 2002. We excluded patients who were diagnosed with AF or atrial flutter (ICD-9-CM codes 427.31 and 427.32 [25]) and under 20 years of age.
A total of 592 non-AF RA patients were included in the study, and 477 patients were females (80.6%), 460 patients were ≤64 year-old (77.7%), and 33.6% had at least one comorbidities. The two most common comorbidities were hypertension (20.8%) and diabetes mellitus (8.3%). The mean follow-up period was 9.97 years (Table 1).

Table 1.
Baseline characteristics of the study participants (N = 592).
2.3. Outcome Measurement and Comorbidities
Patients were followed until first occurrence of ischemic stroke (ICD-9-CM codes 433.x1, 434 (excluding 434.x0), 435, and 436), [28,29] or the last available date in the database. The longest follow-up duration was up to 10 years. The CHA2DS2-VASc score was applied to evaluate the risk of ischemic stroke among RA patients without AF.
The CHADS2 score was calculated according to the following algorithm with five variables: congestive heart failure (ICD-9-CM code 428, 1 point), hypertension (ICD-9-CM codes 401–405, 1 point), age ≥ 75 years (1 point), diabetes (ICD-9-CM code 250, 1 point), and prior ischemic stroke/transient ischemic attack (ICD-9-CM codes 433–438, 2 points). The CHA2DS2-VASc score was calculated according to the following algorithm with 8 variables: Congestive heart failure (ICD-9-CM code 428, 1 point), hypertension (ICD-9-CM codes 401–405, 1 point), diabetes (ICD-9-CM code 250, 1 point), age ≥ 75 years (2 points), prior ischemic stroke/transient ischemic attack (ICD-9-CM codes 433–438, 2 points), vascular disease (ICD-9-CM codes 410.x, 411.0, 412, 420.20–420.24, 429.79, 440.0, 440.20–440.24, 440.29, 440.30–440.32, 440.4, 443.81, 443.89, and 443.9), age 65–75 years (1 point), and female sex (1 point) [24,30,31].
In AF patients, a CHADS2 score ≥2 or a CHA2DS2-VASc score ≥2 was considered as high risk for stroke, respectively [32]. Comorbidities related to ischemic stroke, including hyperlipidemia (ICD-9-CM code 272), chronic obstructive pulmonary disease (ICD-9-CM codes 491, 492, and 496), and chronic kidney disease (ICD-9-CM codes 585, 586, 588.8, and 588.9), and obesity (ICD-9-CM code 278) were included as potential confounding variables [24,30,31,33,34,35,36].
2.4. Statistical Analysis
Baseline characteristics of the study participants are shown as means and standard deviations (SDs) or frequency and percentages for continuous and categorical variables, respectively. Receiver operating characteristic (ROC) curve was constructed to assess the predictive ability of the CHADS2 score and CHA2DS2-VASc score for ischemic stroke. The incidence rates per 10,000 person-years were calculated for ischemic stroke separately for the CHA2DS2-VASc score <2 cohort and the CHA2DS2-VASc score ≥2 cohort. The cumulative incidence of ischemic stroke in the non-AF RA patients for the two cohorts was determined using the Kaplan–Meier method and compared with log-rank test. The incidence rates and incidence rate ratios (IRRs) of the outcome variables, stratified by follow-up periods, between the CHA2DS2-VASc score 0–1 and ≥2 cohorts were calculated using univariate and multiple Poisson regression models (i.e., generalized linear outcome with a Poisson log-liner link function and person-years as the offset variable), adjusting for dyslipidemia, chronic obstructive pulmonary disease, chronic kidney disease, and obesity.
Regarding the model development for the new RA-CHADSV score (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, and vascular disease), we used multiple logistic regression analysis to obtain regression coefficients. The model was validated internally with a bootstrap approach using 1000 samples by resampling with replacement from the original sample with bias-corrected and accelerated confidence interval [37]. The new model of RA-CHADSV score was calculated as the following algorithm: congestive heart failure (1 point), hypertension (1 point), age ≥ 75 years (1 point), diabetes mellitus (1 point), stroke/transient ischemic attack/thromboembolism (1 point), and vascular disease (1 point). All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp, Armonk, NY, USA). A two-tailed p value of <0.05 was considered statistically significant.
3. Results
For the CHA2DS2-VASc score, 396 patients score 0–1, and 196 patients score 2–9. The area under the curve (AUC) of the ROC curve of CHADS2 score was 0.52 (95% CI: 0.42–0.62, p = 0.671) (Figure A1) and that of the CHA2DS2-VASc score was 0.70 (95% CI: 0.61–0.79, p < 0.001) (Figure A2), which showed the ability of the two scores to discriminate ischemic stroke in non-AF RA patients. Figure 1 signifies that non-AF RA patients with CHA2DS2-VASc score ≥2 show significantly higher incidence rates of ischemic stroke compared to those with CHA2DS2-VASc score <2 (p < 0.001).
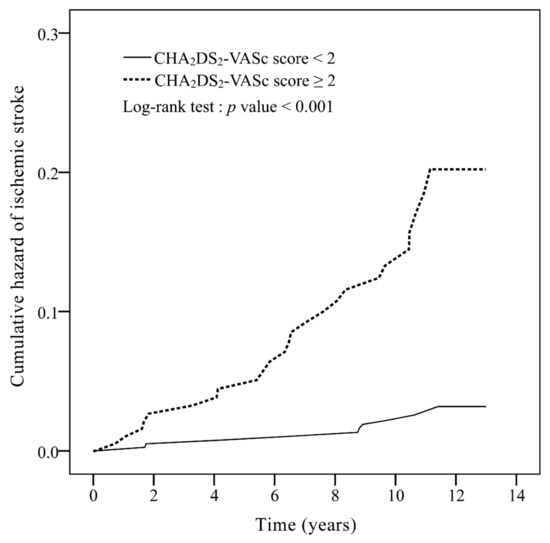
Figure 1.
Cumulative incidence curves of ischemic stroke for rheumatoid arthritis patients without atrial fibrillation, with a CHA2DS2-VASc score 0–1 and CHA2DS2-VASc score ≥2. CHA2DS2-VASc: congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, prior stroke or transient ischemic attack (doubled), vascular disease, age 65–74 years, and female.
Table 2 shows the incidence rates and IRRs of ischemic stroke for patients with CHA2DS2-VASc score 0–1 and ≥2. The incidence rates of ischemic stroke in non-AF RA patients with CHA2DS2-VASc score 0–1 and ≥2 were 23.81 and 146.89 per 10,000 person-years, respectively. A significantly higher incidence of ischemic stroke in patients who scored ≥2 was observed (adjusted IRR 6.00, p < 0.001). Table 2 also shows the incidence rates and IRRs of ischemic stroke with stratification by follow-up years. Significantly higher incidences of ischemic stroke were observed in patients with a CHA2DS2-VASc score ≥2 compared to those with a CHA2DS2-VASc score of 0 or 1. The adjusted IRR was 5.38 (p < 0.001) and 5.91 (p < 0.001) for a follow-up of 5 and 10 years, respectively.

Table 2.
Incidence rates and incidence rate ratio of ischemic stroke for patients with rheumatoid arthritis according to CHA2DS2-VASc score ≥2, with stratification by follow up years (N = 592).
A new and simplified score for improving the prediction of ischemic stroke risk in patients with non-AF RA based on the CHA2DS2-VASc score was developed and shown in Table 3. Based on the results of the logistic model obtained while simultaneously considering clinical convenience, variables with a logistic regression coefficient near zero were omitted from the final model (age 65–74 years and female). In addition, the regression coefficients were round to one significant figure for convenience. For congestive heart failure, we compared the AUC obtained based on a regression coefficient of 1 and 2, and found no differences between the two. Therefore, the variable congestive heart failure was given a score of 1 in the final model. Correspondingly, our new proposed score RA-CHADSV is named based on the acronym of the items in it: congestive heart failure (1 point), hypertension (1 point), age ≥ 75 years (1 point), diabetes mellitus (1 point), stroke/transient ischemic attack/thromboembolism (1 point), and vascular disease (1 point). A RA-CHADSV score ≥1 suggested a higher risk of ischemic stroke [26].

Table 3.
Multiple logistic regression model for the development of the retrospective cohort study was to develop a new score (RA-CHADSV) score.
The AUC of ROC curve of the new RA-CHADSV score was 0.73 (95% CI: 0.64–0.82, p < 0.001) (Figure A3). Patients with RA-CHADSV score ≥1 showed a significantly higher incidence rate of ischemic stroke compared to those who scored 0 (p < 0.001) (Figure 2). Table 4 showed that with every one unit of increment in the RA-CHADSV score, the risk of ischemic stroke increased 2.49 times (p < 0.001). In addition, a significantly higher ischemic stroke incidence in the patients who scored ≥ 1, compared with those who scored 0, was observed with an adjusted IRR of 7.39 (p < 0.001).
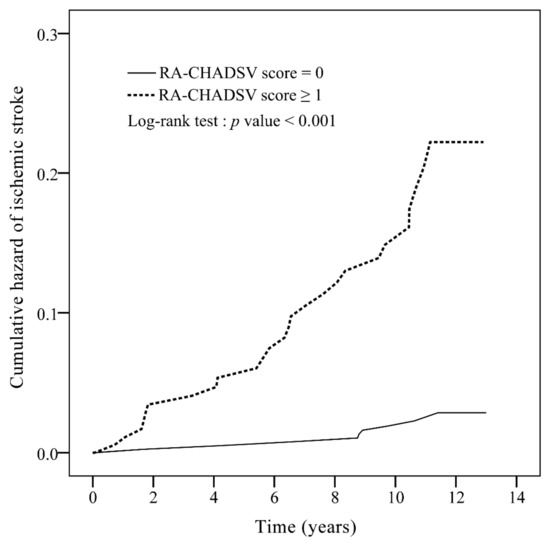
Figure 2.
Cumulative incidence curves of ischemic stroke for rheumatoid arthritis patients without atrial fibrillation, with a RA-CHADSV score 0 and RA-CHADSV score ≥1. RA-CHADSV: rheumatoid arthritis - congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, and vascular disease.

Table 4.
Incidence rates and incidence rate ratio of ischemic stroke for patients with rheumatoid arthritis according to the new RA-CHADSV score (N = 592).
RA-CHADSV score was calculated according to: congestive heart failure (1 point), hypertension (1 point), age ≥ 75 (1 point), diabetes mellitus (1 point), stroke/transient ischemic attack/thromboembolism (1 point), and vascular disease (1 point).
4. Discussion
This retrospective cohort study has three main findings. First, the CHA2DS2-VASc score was able to moderately predict the risk of ischemic stroke in non-AF RA patients, with a score of ≥2 possessed a higher risk of ischemic stroke than patients with a score of 0–1. Second, the CHADS2 score inadequately predicted the risk of ischemic stroke in non-AF RA patients. Third, our newly developed RA-CHADSV score (consisted of six variables with equal weights of one: Congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, and vascular disease) showed similar performance compared with the original CHA2DS2-VASc score in predicting the risk of ischemic stroke in non-AF RA patients. Our study results support the clinical utility of CHA2DS2-VASc score in prediction of the MACE in non-AF RA patients [26]. In addition, we showed that the new RA-CHADSV score was able to predict the risk of ischemic stroke in patients with non-AF RA.
Our results showed that non-AF RA patients possessed a significantly higher risk of ischemic stroke with a follow-up of ten years. The most common comorbidity in our patients was hypertension, which accounted for 33.6% of patients. This result was similar to a previous study where RA patients tended to develop more than one comorbidity within 5 years after diagnosis of RA, with the most common comorbidity was found to be hypertension [38].
The CHA2DS2-VASc score have been used extensively to predict the risk of ischemic stroke in certain individuals [17,20,23,25]. Previous research revealed that patients with RA were at a higher risk for cardiovascular disease compared to patients with other immune-mediated diseases [39,40]. Our finding that non-AF RA patients with a CHA2DS2-VASc score of ≥2 had a higher risk of ischemic stroke is similar to the case of systemic lupus erythematosus patients without AF [25]. Previous studies have also demonstrated an increased risk of ischemic stroke 10 or more years after RA diagnosis [3,4,41]. This result was also observed in our study, especially in patients with a higher CHA2DS2-VASc score (≥2). However, not all the parameters in the CHA2DS2-VASc score can be applicable for patients with RA, hence we developed a new score for non-AF RA patients based on multiple logistic regression analysis with bootstrapping internal validation. The ROC curve of RA-CHADSV and the CHA2DS2-VASc score was 0.73 and 0.70, respectively. Despite the simplicity (only six variables of equal weights) of our new model, the performance was comparable with the original CHA2DS2-VASc score in predicting ischemic stroke risk among non-AF RA patients.
This study has a few limitations that should be noted. Patient’s physical activity, lifestyle factors, and laboratory data were not available in the LHID, and therefore could not be adjusted in the statistical models. The model for the new RA-CHADV score was validated internally using bootstrap method, which should be externally validated in future studies.
5. Conclusions
Findings from this retrospective cohort study showed that CHA2DS2-VASc score was able to moderately predict the risk of ischemic stroke in non-AF RA patients. Furthermore, a new RA-CHADSV score was developed in this study, which was based on six variables, including congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, and vascular disease. This simple tool could be used to identify non-AF RA patients with an increased risk of ischemic stroke, and to help physicians in the management of these patients.
Author Contributions
Conceptualization, K.-J.N. and M.-C.L.; methodology, M.-C.L.; formal analysis, C.-W.H.; writing—original draft preparation, K.-J.N., C.-W.H., and M.-C.L.; writing—review and editing, M.-C.L. and M.K. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by the National Health Research Institutes, Taiwan. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare or the National Health Research Institutes, Taiwan.
Conflicts of Interest
The authors declare there are no conflicts of interest.
Appendix A
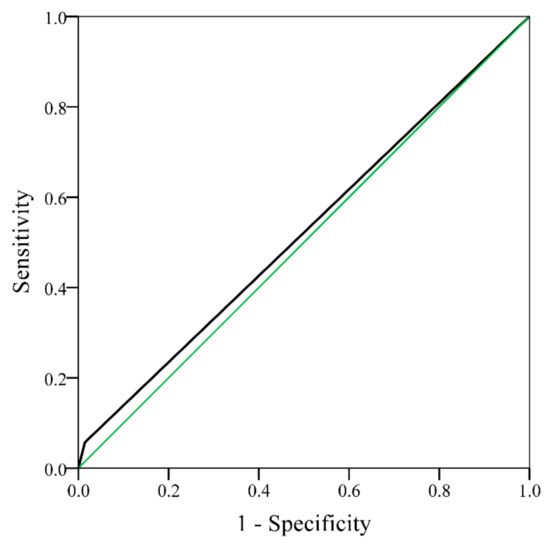
Figure A1.
Receiver operating characteristic curve of ischemic stroke for rheumatoid arthritis patients without atrial fibrillation, with a CHADS2 score 0–1 and CHADS2 score ≥2 (area under the curve = 0.52, 95% CI 0.42–0.62, p = 0.671). CHADS2: congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and stroke [doubled]); CI: confidence interval.
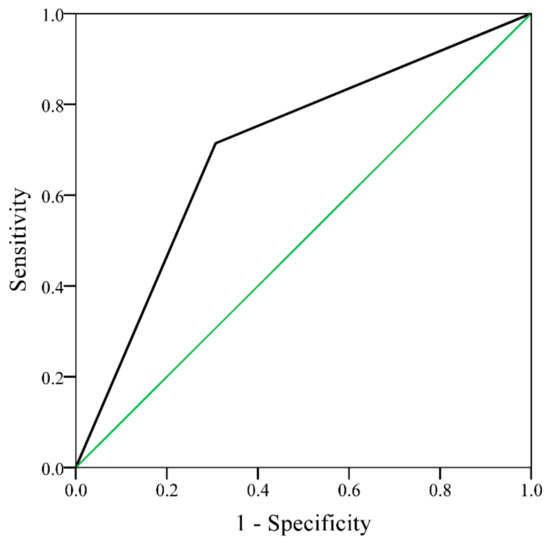
Figure A2.
Receiver operating characteristic curve of ischemic stroke for rheumatoid arthritis patients without atrial fibrillation, with a CHA2DS2 score 0–1 and CHA2DS2 score ≥2 (area under the curve = 0.70, 95% CI 0.61–0.79, p < 0.001). CHA2DS2-VASc: congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, stroke/transient ischemic attack (doubled), vascular disease, age 65–74 years, and female; CI: confidence interval.
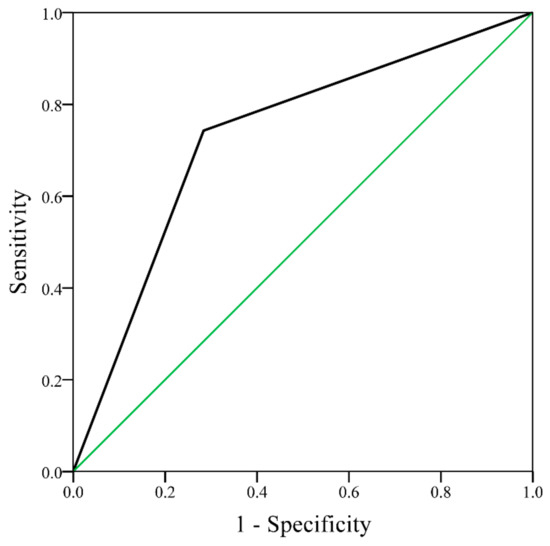
Figure A3.
Receiver operating characteristic curve of ischemic stroke for rheumatoid arthritis patients without atrial fibrillation, with a RA-CHADSV score 0 and RA-CHADSV score ≥1 (area under the curve = 0.73, 95% CI 0.64–0.82, p < 0.001). RA-CHADSV: congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, and vascular disease; CI: confidence interval.
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.W.; Chen, C.M.; Leong, P.Y.; Chen, C.H.; Li, Y.C.; Wang, Y.H.; Lin, L.C.; Chiou, J.Y.; Wei, J.C. Methotrexate might reduce ischemic stroke in patients with rheumatoid arthritis: A population-based retrospective cohort study. Int. J. Rheum. Dis. 2018, 21, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Lindhardsen, J.; Ahlehoff, O.; Gislason, G.H.; Madsen, O.R.; Olesen, J.B.; Svendsen, J.H.; Torp-Pedersen, C.; Hansen, P.R. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ 2012, 344, e1257. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.J.; Ralston, S.H.; Wardlaw, J.M. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke 2016, 47, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Oghalai, T.U.; Wu, H.; McNearney, T.A.; Granger, C.V.; Ottenbacher, K.J. Functional outcome after stroke in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 2008, 59, 984–988. [Google Scholar] [CrossRef]
- Zinger, H.; Sherer, Y.; Shoenfeld, Y. Atherosclerosis in autoimmune rheumatic diseases-mechanisms and clinical findings. Clin. Rev. Allergy Immunol. 2009, 37, 20–28. [Google Scholar] [CrossRef]
- Sherer, Y.; Shoenfeld, Y. Mechanisms of disease: Atherosclerosis in autoimmune diseases. Nat. Clin. Pract. Rheumatol. 2006, 2, 99–106. [Google Scholar] [CrossRef]
- Skeoch, S.; Bruce, I.N. Atherosclerosis in rheumatoid arthritis: Is it all about inflammation? Nat. Rev. Rheumatol. 2015, 11, 390–400. [Google Scholar] [CrossRef]
- Tiosano, S.; Yavne, Y.; Gendelman, O.; Watad, A.; Comaneshter, D.; Shoenfeld, Y.; Cohen, A.D.; Amital, D. Stroke among rheumatoid arthritis patients: does age matter? A real-life study. Neuroepidemiology 2017, 49, 99–105. [Google Scholar] [CrossRef]
- Masuda, H.; Miyazaki, T.; Shimada, K.; Tamura, N.; Matsudaira, R.; Yoshihara, T.; Ohsaka, H.; Sai, E.; Matsumori, R.; Fukao, K.; et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J. Cardiol. 2014, 64, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Dadonienė, J.; Stropuvienė, S.; Stukas, R.; Venalis, A.; Sokka-Isler, T. Predictors of mortality in patients with rheumatoid arthritis in Lithuania: Data from a cohort study over 10 years. Medicina Kaunas 2015, 51, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, P.S.; Kabra, R. Stroke risk in atrial fibrillation: Beyond the CHA2DS2-VASc score. Curr. Cardiol. Rep. 2019, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, A.; Podgorski, M.; Wozniak, T.; Stefanczyk, L.; Strzelecki, M. Computational fluid dynamics as an engineering tool for the reconstruction of hemodynamics after carotid artery stenosis operation: A case study. Medicina Kaunas 2018, 54, 42. [Google Scholar] [CrossRef]
- Zhu, W.G.; Xiong, Q.M.; Hong, K. Meta-analysis of CHADS2 versus CHA2DS2-VASc for predicting stroke and thromboembolism in atrial fibrillation patients independent of anticoagulation. Tex. Heart Inst. J. 2015, 42, 6–15. [Google Scholar] [CrossRef]
- Lin, L.Y.; Lee, C.H.; Yu, C.C.; Tsai, C.T.; Lai, L.P.; Hwang, J.J.; Chen, P.C.; Lin, J.L. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation--a nation wide database analysis. Atherosclerosis 2011, 217, 292–295. [Google Scholar] [CrossRef]
- Chao, T.F.; Liu, C.J.; Tuan, T.C.; Chen, S.J.; Wang, K.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chen, T.J.; et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: Which scoring system should be used for Asians? Heart Rhythm 2016, 13, 46–53. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Ng, C.Y.; Li, G.; Liu, T. Meta-analysis of CHADS2 score in predicting atrial fibrillation. Am. J. Cardiol. 2015, 116, 554–562. [Google Scholar] [CrossRef]
- Sugioka, K.; Fujita, S.; Iwata, S.; Ito, A.; Matsumura, Y.; Hanatani, A.; Doi, A.; Takagi, M.; Naruko, T.; Ueda, M.; et al. Relationship between CHADS2 score and complex aortic plaques by transesophageal echocardiography in patients with nonvalvular atrial fibrillation. Ultrasound Med. Biol. 2014, 40, 2358–2364. [Google Scholar] [CrossRef]
- Senoo, K.; Lane, D.A.; Lip, G.Y. Stroke risk reduction with oral anticoagulation using CHA2DS2-VASc in a Japanese AF population: A modeling analysis. Int. J. Cardiol. 2015, 181, 247–254. [Google Scholar] [CrossRef]
- Chen, Y.L.; Cheng, C.L.; Huang, J.L.; Yang, N.I.; Chang, H.C.; Chang, K.C.; Sung, S.H.; Shyu, K.G.; Wang, C.C.; Yin, W.H.; et al. Mortality prediction using CHADS2/CHA2DS2-VASc/R2CHADS2 scores in systolic heart failure patients with or without atrial fibrillation. Medicine Baltimore 2017, 96, e8338. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Lip, G.Y.; Makaritsis, K.; Papavasileiou, V.; Vemmou, A.; Koroboki, E.; Savvari, P.; Manios, E.; Milionis, H.; Vemmos, K. CHADS₂, CHA₂S₂DS₂-VASc, and long-term stroke outcome in patients without atrial fibrillation. Neurology 2013, 80, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Melgaard, L.; Gorst-Rasmussen, A.; Lane, D.A.; Rasmussen, L.H.; Larsen, T.B.; Lip, G.Y. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA 2015, 314, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, Y.H.; Hsieh, T.F.; Chuang, S.Y.; Wu, M.J. Prediction of mortality in incident hemodialysis patients: a validation and comparison of CHADS2, CHA2DS2, and CCI scores. PLoS ONE 2016, 11, e0154627. [Google Scholar] [CrossRef]
- Hu, W.S.; Lin, C.L. CHA2DS2-VASc score for prediction of ischemic stroke in patients with systemic lupus erythematosus without atrial fibrillation. Lupus 2018, 27, 1240–1246. [Google Scholar] [CrossRef]
- Cioffi, G.; Viapiana, O.; Orsolini, G.; Idolazzi, L.; Fracassi, E.; Ognibeni, F.; Dalbeni, A.; Gatti, D.; Carletto, A.; Fassio, A.; et al. Usefulness of CHA2 DS2 -VASc score to predict mortality and hospitalization in patients with inflammatory arthritis. Int. J. Rheum. Dis. 2020, 23, 106–115. [Google Scholar] [CrossRef]
- National Health Insurance Administration. Available online: https://ws.nhi.gov.tw/001/Upload/%20293/RelFile/Ebook/English.pdf (accessed on 28 January 2020).
- Cozzolino, F.; Abraha, I.; Orso, M.; Mengoni, A.; Cerasa, M.F.; Eusebi, P.; Ambrosio, G.; Montedori, A. Protocol for validating cardiovascular and cerebrovascular ICD-9-CM codes in healthcare administrative databases: The umbria data value project. BMJ Open 2017, 7, e013785. [Google Scholar] [CrossRef]
- Chen, Y.R.; Hsieh, F.I.; Lien, L.M.; Hu, C.J.; Jeng, J.S.; Peng, G.S.; Tang, S.C.; Chi, N.F.; Sung, Y.F.; Chiou, H.Y. Rheumatoid arthritis significantly increased recurrence risk after ischemic stroke/transient ischemic attack. J. Neurol. 2018, 265, 1810–1818. [Google Scholar] [CrossRef]
- Chua, S.K.; Shyu, K.G.; Lu, M.J.; Hung, H.F.; Cheng, J.J.; Chiu, C.Z.; Lin, C.H.; Chao, H.H.; Lo, H.M. Renal dysfunction and the risk of postoperative atrial fibrillation after cardiac surgery: Role beyond the CHA2DS2-VASc score. Europace 2015, 17, 1363–1370. [Google Scholar] [CrossRef]
- Hu, W.S.; Lin, C.L. Postoperative ischemic stroke and death prediction with CHA2DS2-VASc score in patients having coronary artery bypass grafting surgery: a nationwide cohort study. Int. J. Cardiol. 2017, 241, 120–123. [Google Scholar] [CrossRef]
- Chiang, C.E.; Wu, T.J.; Ueng, K.C.; Chao, T.F.; Chang, K.C.; Wang, C.C.; Lin, Y.J.; Yin, W.H.; Kuo, J.Y.; Lin, W.S.; et al. 2016 Guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J. Formos. Med. Assoc. 2016, 115, 893–952. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Lensmar, C.; Ingelsson, E.; Bäck, M. Differential association of chronic obstructive pulmonary disease with myocardialinfarction and ischemic stroke in a nation-wide cohort. Int. J. Cardiol. 2014, 173, 601–603. [Google Scholar] [CrossRef] [PubMed]
- El Husseini, N.; Kaskar, O.; Goldstein, L.B. Chronic kidney disease and stroke. Adv. Chronic Kidney Dis. 2014, 21, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Van Rooy, M.J.; Pretorius, E. Obesity, hypertension and hypercholesterolemia as risk factors for atherosclerosis leading to ischemic events. Curr. Med. Chem. 2014, 21, 2121–2129. [Google Scholar] [CrossRef]
- Tziomalos, K.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Dyslipidemia as a risk factor for ischemic stroke. Curr. Top. Med. Chem. 2009, 9, 1291–1297. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Harrell, F.E., Jr.; Borsboom, G.J.; Eijkemans, M.J.; Vergouwe, Y.; Habbema, J.D. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef]
- Innala, L.; Sjoberg, C.; Moller, B.; Ljung, L.; Smedby, T.; Sodergren, A.; Magnusson, S.; Rantapaa-Dahlqvist, S.; Wallberg-Jonsson, S. Co-morbidity in patients with early rheumatoid arthritis - inflammation matters. Arthritis Res. Ther. 2016, 18, 33. [Google Scholar] [CrossRef]
- Baena-Diez, J.M.; Garcia-Gil, M.; Comas-Cufi, M.; Ramos, R.; Prieto-Alhambra, D.; Salvador-Gonzalez, B.; Elosua, R.; Degano, I.R.; Penafiel, J.; Grau, M. Association between chronic immune-mediated inflammatory diseases and cardiovascular risk. Heart 2018, 104, 119–126. [Google Scholar] [CrossRef]
- Zha, A.M.; Di Napoli, M.; Behrouz, R. Prevention of stroke in rheumatoid arthritis. Curr. Neurol. Neurosci. Rep. 2015, 15, 77. [Google Scholar] [CrossRef]
- Holmqvist, M.; Gransmark, E.; Mantel, A.; Alfredsson, L.; Jacobsson, L.T.; Wallberg-Jonsson, S.; Askling, J. Occurrence and relative risk of stroke in incident and prevalent contemporary rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 541–546. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).