Effects of Maximal Strength Training on Perceived-Fatigue and Functional Mobility in Persons with Relapsing-Remitting Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
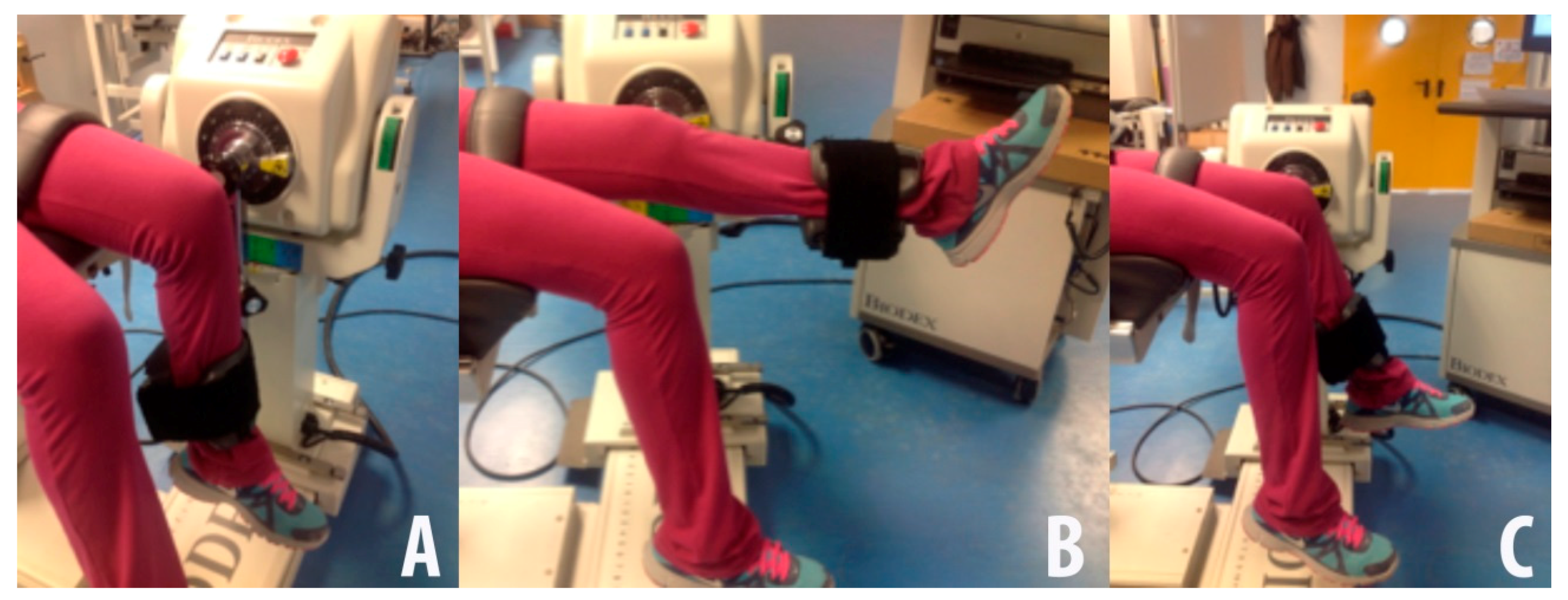
2.2.1. Isokinetic and Isometric Strength Measurements
2.2.2. Perceived-Fatigue
2.2.3. Evaluation of Functional Mobility
2.2.4. Intervention
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krupp, L.B.; Alvarez, L.A.; Larocca, N.G.; Scheinberg, L.C. Fatigue in multiple sclerosis. Arch. Neurol. 1988, 45, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.; van der Mei, I.A.F.; Ponsonby, A.L.; Pittas, F.; Quinn, S.; Dwyer, T.; Lucas, R.M.; Taylor, B.V. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult. Scler. 2013, 19, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.S.; Chalah, M.A. Fatigue in multiple sclerosis. Insights into evaluation and management. Neurophysiol. Clin. 2017, 47, 139–171. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Bush, S.; Gappmaier, E. Associations between fatigue and disability, functional mobility, depression, and quality of life in people with multiple sclerosis. Int. J. MS Care. 2016, 18, 71–77. [Google Scholar] [CrossRef]
- Pilutti, L.A.; Greenlee, T.A.; Motl, R.W.; Nickrent, M.S.; Petruzzello, S.J. Effects of exercise training on fatigue in multiple sclerosis: A meta-analysis. Psychosom. Med. 2013, 75, 575–580. [Google Scholar] [CrossRef]
- Pilutti, L.A.; Platta, M.E.; Motl, R.W.; Latimer-Cheung, A.E. The safety of exercise training in multiple sclerosis: A systematic review. J. Neurol. Sci. 2014, 343, 3–7. [Google Scholar] [CrossRef]
- Heine, M.; van de Port, I.; Rietberg, M.B.; van Wegen, E.E.H.; Kwakkel, G. Exercise therapy for fatigue in multiple sclerosis. Cochrane. Database. Syst. Rev. 2015, 9, CD009956. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Martin-Ginis, K.A.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828. [Google Scholar] [CrossRef]
- Cruickshank, T.M.; Reyes, A.R.; Ziman, M.R. A systematic review and meta-analysis of strength training in individuals with multiple sclerosis or parkinson disease. Medicine 2015, 94, e411. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E.; Lund, C.; Rasmussen, C.; Petersen, T.; Sørensen, H.; Ingemann-Hansen, T.; Overgaard, K. Neural drive increases following resistance training in patients with multiple sclerosis. J. Neurol. 2013, 260, 1822–1832. [Google Scholar] [CrossRef]
- Motl, R.W.; Pilutti, L.A.; Sandroff, B.M.; Klaren, R.; Balantrapu, S.; McAuley, E.; Sosnoff, J.J.; Fernhall, B. Rationale and design of a randomized controlled, clinical trial investigating a comprehensive exercise stimulus for improving mobility disability outcomes in persons with multiple sclerosis. Contemp. Clin. Trials. 2013, 35, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.; Dieberg, G.; Smart, N. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: A meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Martinez, G.; Cereatti, A.; Della Croce, U.; Ventura, L.; Dvir, Z.; Deriu, F. Isokinetic predictors of gait speed increase following high-intensity resistance training of the ankle dorsiflexors in people with multiple sclerosis: A pilot study. Clin. Biomech. 2019, 67, 102–106. [Google Scholar] [CrossRef]
- Skjerbæk, A.G.; Møller, A.B.; Jensen, E.; Vissing, K.; Sørensen, H.; Nybo, L.; Stenager, E.; Dalgas, U. Heat sensitive persons with multiple sclerosis are more tolerant to resistance exercise than to endurance exercise. Mult. Scler. J. 2013, 19, 932–940. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; McCoy, S.C.; Castellano, V.; Gutierrez, G.; Stevens, J.E.; Walter, G.A.; Vandenborne, K. Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult. Scler. 2004, 10, 668–674. [Google Scholar] [CrossRef]
- Duchateau, J.; Semmler, J.G.; Enoka, R.M. Training adaptations in the behavior of human motor units. J. Appl. Physiol. 2006, 101, 1766–1775. [Google Scholar] [CrossRef]
- Karpatkin, H.I.; Cohen, E.T.; Klein, S.; Park, D.; Wright, C.; Zervas, M. The effect of maximal strength training on strength, walking, and balance in people with multiple sclerosis: A pilot study. Mult. Scler. Int. 2016, 2016, 5235971. [Google Scholar] [CrossRef]
- Kierkegaard, M.; Olsson, T.; Johansson, S.; Lundberg, I.E.; Ygberg, S.; Opava, C.; Holmqvist, L.W.; Piehl, F. High-intensity resistance training in multiple sclerosis. An exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J. Neurol. Sci. 2016, 362, 251–257. [Google Scholar] [CrossRef]
- Heesen, C.; Nawrath, L.; Reich, C.; Bauer, N.; Schulz, K.H.; Gold, S.M. Fatigue in multiple sclerosis: An example of cytokine mediated sickness behaviour? J. Neurol. Neurosurg. Psychiatry. 2006, 77, 34–39. [Google Scholar] [CrossRef]
- DeBolt, L.S.; McCubbin, J.A. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch. Phys. Med. Rehabil. 2004, 85, 290–297. [Google Scholar] [CrossRef]
- Krupp, L.B.; Larocca, N.G.; Muir Nash, J.; Steinberg, A.D. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, E.; Sandroff, B.M.; Learmonth, Y.C.; Motl, R.W. Validity of the Timed Up and Go test as a measure of functional mobility in persons with multiple sclerosis. Arch. Phys. Med. Rehabil. 2016, 97, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Regola, A.; Meotti, M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil. Rehabil. 2006, 28, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Fimland, M.S.; Helgerud, J.; Gruber, M.; Leivseth, G.; Hoff, J. Enhanced neural drive after maximal strength training in multiple sclerosis patients. Eur. J. Appl. Physiol. 2010, 110, 435–443. [Google Scholar] [CrossRef]
- Kjølhede, T.; Vissing, K.; Dalgas, U. Multiple sclerosis and progressive resistance training: A systematic review. Mult. Scler. J. 2012, 18, 1215–1228. [Google Scholar] [CrossRef]
- Kemmler, W.K.; Lauber, D.; Wassermann, A.; Mayhew, J.L. Predicting maximal strength in trained postmenopausal woman. J. Strength. Cond. Res. 2006, 20, 838–842. [Google Scholar]
- Knutzen, K.; Brilla, L.; Caine, D. Validity of 1RM prediction equations for older adults. J. Strength. Cond. Res. 1999, 13, 242–246. [Google Scholar]
- Pierce, C.A.; Block, R.A.; Aguinis, H. Cautionary note on reporting eta-squared values from multifactor ANOVA designs. Educ. Psychol. Measur. 2004, 64, 916–924. [Google Scholar] [CrossRef]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: San Diego, CA, USA, 1995; p. 75106. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Erlbaum Associates: Hillsdale, NJ, USA, 1988; p. 815. [Google Scholar]
- Moss-Morris, R.; Harrison, A.; Safari, R.; Norton, S.; van der Linden, M.; Picariello, F.; Thomas, S.; White, C.C.; Mercer, T. Which behavioural and exercise interventions targeting fatigue show the most promise in multiple sclerosis? A systematic review with narrative synthesis and meta. Behav. Res. Ther. 2019, in press. [Google Scholar] [CrossRef]
- Yang, T.T.; Wang, L.; Deng, X.Y.; Yu, G. Pharmacological treatments for fatigue in patients with multiple sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 380, 256–261. [Google Scholar] [CrossRef]
- Kjølhede, T.; Vissing, K.; Langeskov-Christensen, D.; Stenager, E.; Petersen, T.; Dalgas, U. Relationship between muscle strength parameters and functional capacity in persons with mild to moderate degree multiple sclerosis. Mult. Scler. Relat. Disord. 2015, 4, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, D.; Guclu-Gunduz, A.; Yazici, G.; Lambeck, J.; Batur-Caglayan, H.Z.; Irkec, C.; Nazliel, B. Effects of Ai-Chi on balance, functional mobility, strength and fatigue in patients with multiple sclerosis: A pilot study. NeuroRehabilitation 2013, 33, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.J.; Taylor, N.F.; Shields, N.; Prasad, D.; McDonald, E.; Gillon, A. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: A randomized controlled trial. Mult. Scler. J. 2011, 17, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Medina-Perez, C.; de Souza-Teixeira, F.; Fernandez-Gonzalo, R.; de Paz-Fernandez, J.A. Effects of a resistance training program and subsequent detraining on muscle strength and muscle power in multiple sclerosis patients. NeuroRehabilitation 2014, 34, 523–530. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E.; Jakobsen, J.; Petersen, T.; Hansen, H.J.; Knudsen, C.; Overgaard, K.; Ingemann-Hansen, T. Fatigue, mood and quality of life improve in multiple sclerosis patients after progressive resistance training. Mult. Scler. 2010, 16, 480–490. [Google Scholar] [CrossRef]
- Mohr, D.C.; Hart, S.L.; Goldberg, A. Effects of treatment for depression on fatigue in multiple sclerosis. Psychosom. Med. 2003, 65, 542–547. [Google Scholar] [CrossRef]
- Strober, L.B.; Becker, A.; Randolph, J.J. Role of positive lifestyle activities on mood, cognition, well-being, and disease characteristics in multiple sclerosis. Appl. Neuropsychol. Adult 2018, 25, 304–311. [Google Scholar] [CrossRef]
- Eng, J.J.; Reime, B. Exercise for depressive symptoms in stroke patients: A systematic review and meta-analysis. Clin. Rehabil. 2014, 28, 731–739. [Google Scholar] [CrossRef]
- Motl, R.W. Lifestyle physical activity in persons with multiple sclerosis: The new kid on the MS block. Mult. Scler. J. 2014, 20, 1025–1029. [Google Scholar] [CrossRef]
| Week | %1RM | Sets | Repetitions | Rest Interval (min) | |
|---|---|---|---|---|---|
| Pre-test | 0 | Pre-Intervention measurements | |||
| Conditioning period | 1–2 | 50 | 2 | 8–10 | 3 |
| 3–4 | 60 | 2 | 12–14 | 3 | |
| Maximal strength training period | 5–6 | 75 | 3 | 7 | 5 |
| 1RM measurements for adjusting training loads | |||||
| 7–8 | 80 | 4 | 6 | 5 | |
| 9–10 | 85 | 4 | 4 | 5 | |
| 11–12 | 90 | 5 | 4 | 5 | |
| Post-test | 13 | Post-Intervention measurements | |||
| Follow-up | 22 | Detraining measurements | |||
| Week | Day | Exercise |
|---|---|---|
| 1–4 | Monday Tuesday Thursday | Dumbbell Shoulder Press Cable Standing Biceps Curl Cable Triceps Pushdown Chest Press Machine Wide-Grip Lat Pulldown Leg Extension Leg Curl Multipower Standing Calf Raises |
| 5–12 | 1st | Chest Press Machine Barbell Incline Bench Press Leg Curl Leg Extension Multipower Standing Calf Raises |
| 2nd | Alternate Hammer Curl Cable Triceps Pushdown Front Dumbell Raise Side Lateral Raise | |
| 3rd | Wide-Grip Lat Pulldown Seated Cable Rows Leg Press Thigh Adductor Half Stance Multipower Squat |
| MST Group | Control Group | p | dg | |||
|---|---|---|---|---|---|---|
| Women/Men | 9/4 | 12/1 | -- | -- | ||
| Age (years) | 45.31 | (11.06) | 41.31 | (9.58) | 0.460 | 0.37 |
| Body mass (kg) | 66.02 | (15.21) | 58.82 | (11.31) | 0.320 | 0.52 |
| EDSS (unitless) | 2.38 | (0.98) | 2.81 | (1.33) | 0.429 | −0.36 |
| FSS (unitless) | 57.08 | (5.92) | 52.46 | (7.08) | 0.084 | 0.69 |
| TUG (s) | 7.06 | (1.40) | 7.35 | (2.10) | 0.720 | −0.16 |
| Pre-Test (1) | Post-Test (2) | Follow-Up (3) | F | p | ŋp2 | Dif 1‒2 (%) | dg (1‒2) | Dif 1‒3 (%) | dg (1‒3) | Dif 2‒3 (%) | dg (2‒3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QPTIK (Nm/kg) | MST | 2.96 | (0.72) | 3.44 | (0.85) | 3.11 | (0.81) | 80.95 | <0.001 | 0.87 | Δ16.22 | −0.62 ** | Δ5.07 | −0.20 | ∇9.59 | 0.36 ** |
| CG | 3.07 | (0.98) | 2.60 | (1.13) | 2.62 | (1.05) | 7.53 | 0.018 | 0.39 | ∇15.31 | 0.45 ** | ∇14.66 | 0.43 ** | Δ0.77 | −0.02 | |
| HPTIK (Nm/kg) | MST | 1.32 | (0.36) | 1.71 | (0.32) | 1.62 | (0.45) | 23.47 | <0.001 | 0.55 | Δ29.55 | −1.01 ** | Δ22.73 | −0.78 ** | ∇5.26 | 0.26 |
| CG | 1.55 | (0.52) | 1.55 | (0.58) | 1.53 | (0.49) | 0.03 | 0.879 | 0.01 | 0.00 | -- | ∇1.29 | 0.04 | ∇1.29 | 0.03 | |
| QPTIM (Nm/kg) | MST | 4.26 | (1.01) | 4.98 | (1.16) | 4.29 | (1.20) | 44.17 | <0.001 | 0.79 | Δ16.90 | −0.67 ** | Δ0.70 | −0.03 | ∇13.86 | 0.56 ** |
| CG | 4.07 | (1.54) | 3.75 | (1.32) | 3.71 | (1.45) | 0.93 | 0.355 | 0.07 | ∇7.86 | 0.19 | ∇8.85 | 0.22 | ∇1.07 | 0.03 | |
| HPTIM (Nm/kg) | MST | 1.71 | (0.36) | 1.82 | (0.31) | 1.74 | (0.26) | 18.37 | 0.001 | 0.61 | Δ6.43 | −0.29 * | Δ1.75 | −0.08 | ∇4.40 | 0.24 |
| CG | 1.87 | (0.75) | 1.74 | (0.74) | 1.82 | (0.73) | 1.98 | 0.184 | 0.14 | ∇6.95 | 0.16 | ∇2.67 | 0.06 | Δ4.60 | −0.10 | |
| FSS (points) | MST | 57.08 | (5.92) | 22.85 | (6.18) | 48.46 | (9.49) | 145.44 | <0.001 | 0.87 | Δ59.97 | 5.41 ** | Δ15.10 | 1.36 * | ∇112.08 | −3.88 ** |
| CG | 52.46 | (7.08) | 50.54 | (9.71) | 51.69 | (6.64) | 0.79 | 0.393 | 0.06 | Δ3.66 | 0.25 | Δ1.47 | 0.10 | ∇2.28 | −0.11 | |
| TUG (s) | MST | 7.06 | (1.40) | 5.67 | (0.84) | 6.63 | (1.12) | 46.94 | <0.001 | 0.80 | Δ19.69 | 0.93 ** | Δ6.09 | 0.29 | ∇16.93 | −1.07** |
| CG | 7.35 | (2.15) | 7.15 | (1.99) | 8.09 | (2.34) | 4.10 | 0.066 | 0.25 | Δ2.72 | 0.09 | ∇10.07 | −0.32 | ∇13.15 | −0.44 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Illan, R.; Reina, R.; Barbado, D.; Sabido, R.; Moreno-Navarro, P.; Roldan, A. Effects of Maximal Strength Training on Perceived-Fatigue and Functional Mobility in Persons with Relapsing-Remitting Multiple Sclerosis. Medicina 2020, 56, 718. https://doi.org/10.3390/medicina56120718
Gomez-Illan R, Reina R, Barbado D, Sabido R, Moreno-Navarro P, Roldan A. Effects of Maximal Strength Training on Perceived-Fatigue and Functional Mobility in Persons with Relapsing-Remitting Multiple Sclerosis. Medicina. 2020; 56(12):718. https://doi.org/10.3390/medicina56120718
Chicago/Turabian StyleGomez-Illan, Ramon, Raul Reina, David Barbado, Rafael Sabido, Pedro Moreno-Navarro, and Alba Roldan. 2020. "Effects of Maximal Strength Training on Perceived-Fatigue and Functional Mobility in Persons with Relapsing-Remitting Multiple Sclerosis" Medicina 56, no. 12: 718. https://doi.org/10.3390/medicina56120718
APA StyleGomez-Illan, R., Reina, R., Barbado, D., Sabido, R., Moreno-Navarro, P., & Roldan, A. (2020). Effects of Maximal Strength Training on Perceived-Fatigue and Functional Mobility in Persons with Relapsing-Remitting Multiple Sclerosis. Medicina, 56(12), 718. https://doi.org/10.3390/medicina56120718







