CT Urography Findings of Upper Urinary Tract Carcinoma and Its Mimickers: A Pictorial Review
Abstract
1. Introduction
2. CTU Technique
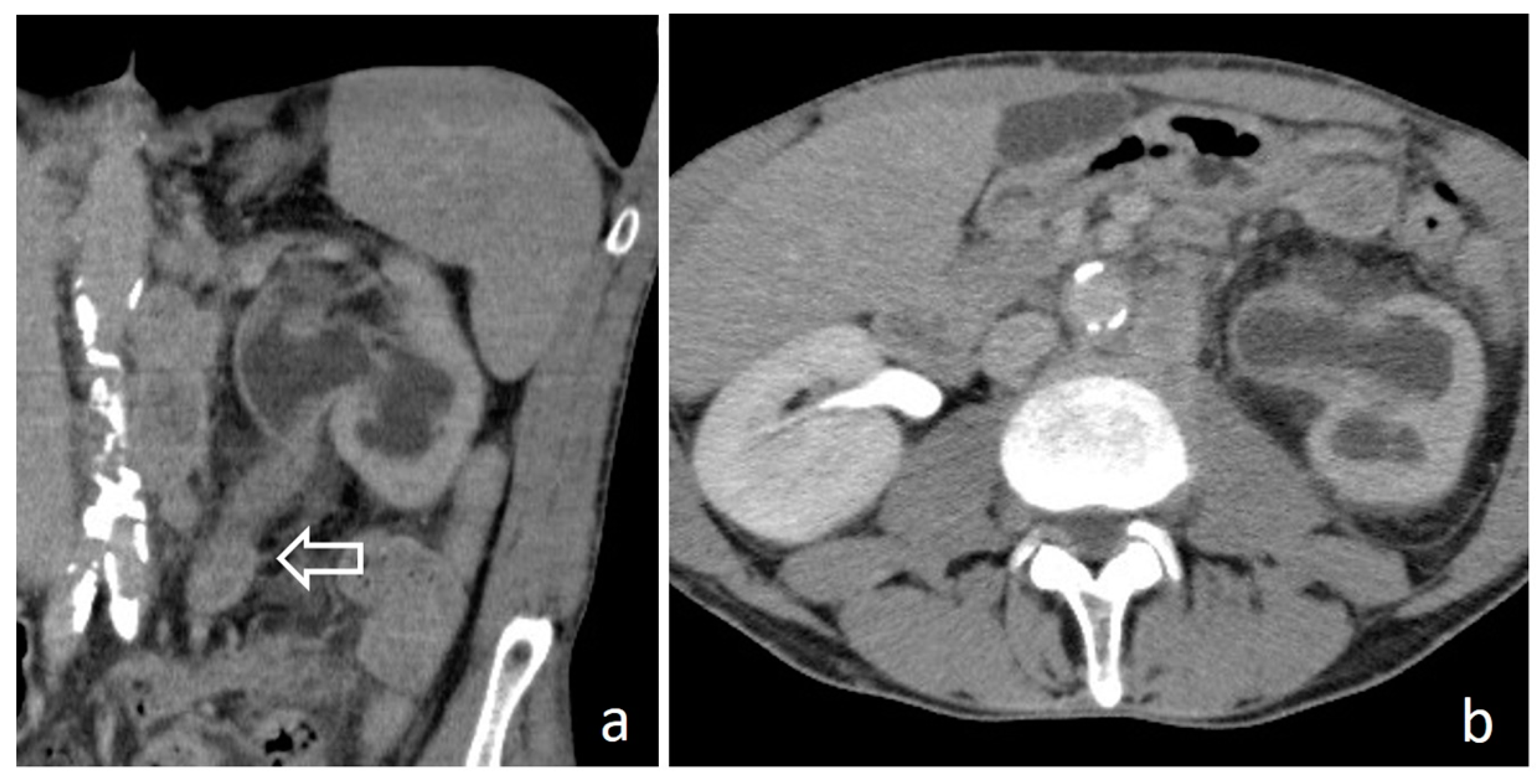
3. Imaging Findings
4. Mimickers of UTUC
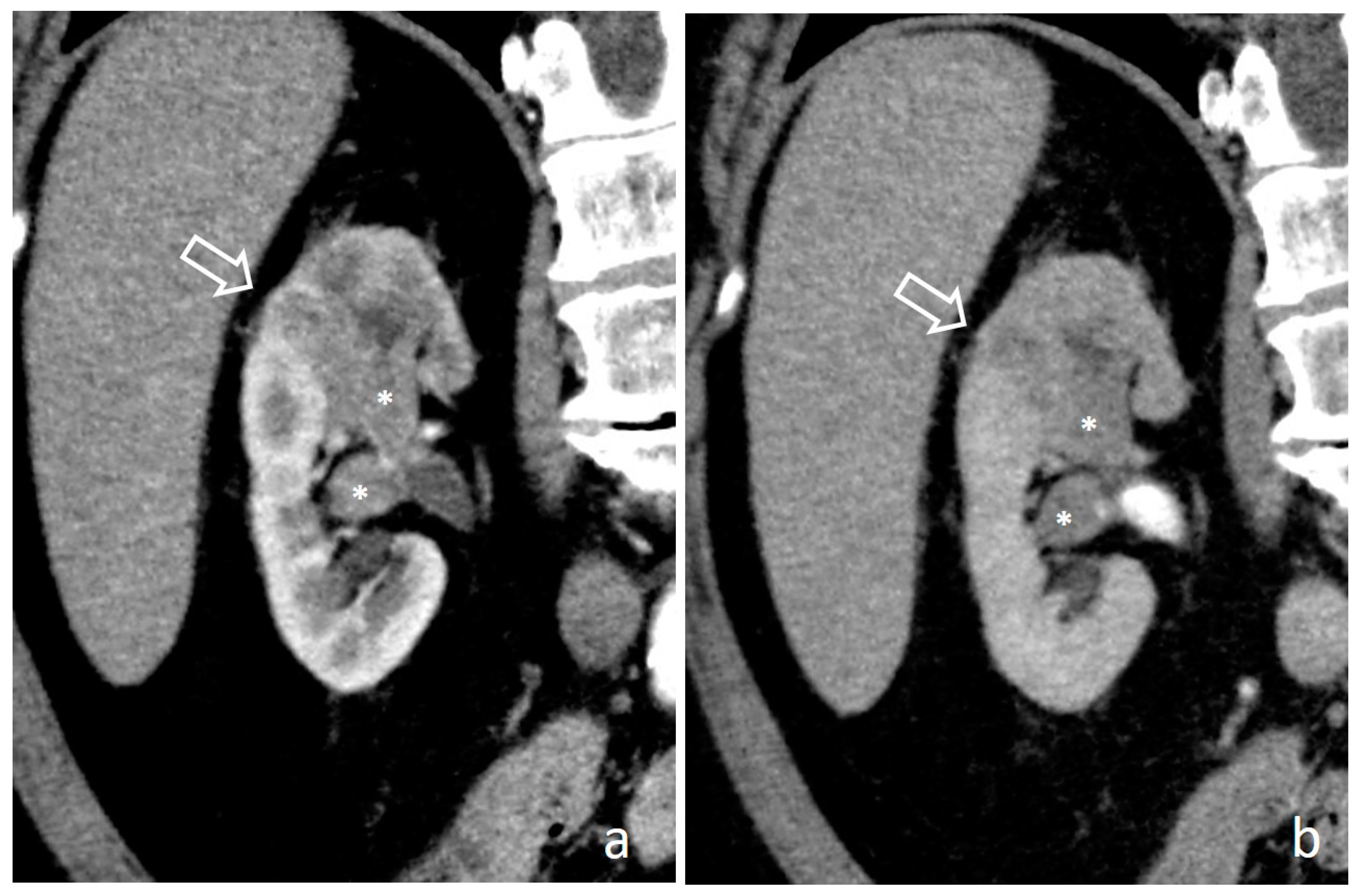
4.1. Hypertrophied Papilla
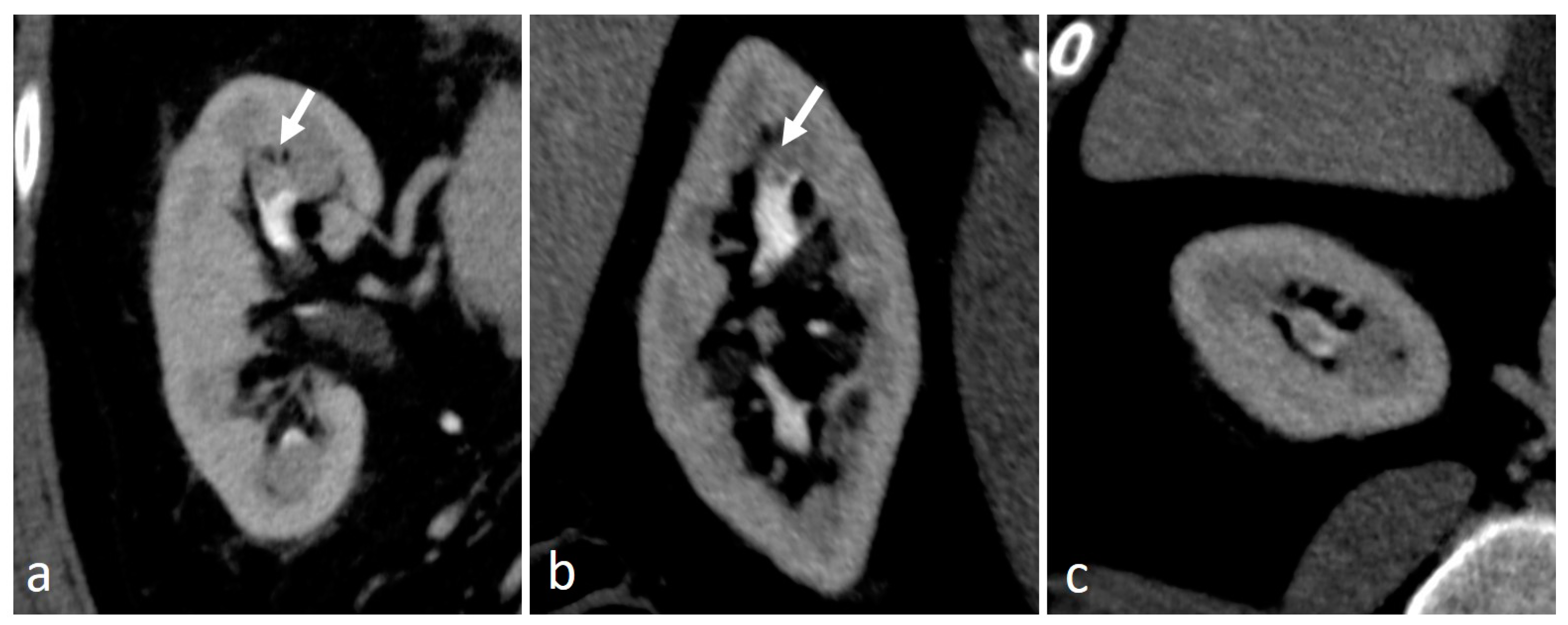
4.2. Blood Clots
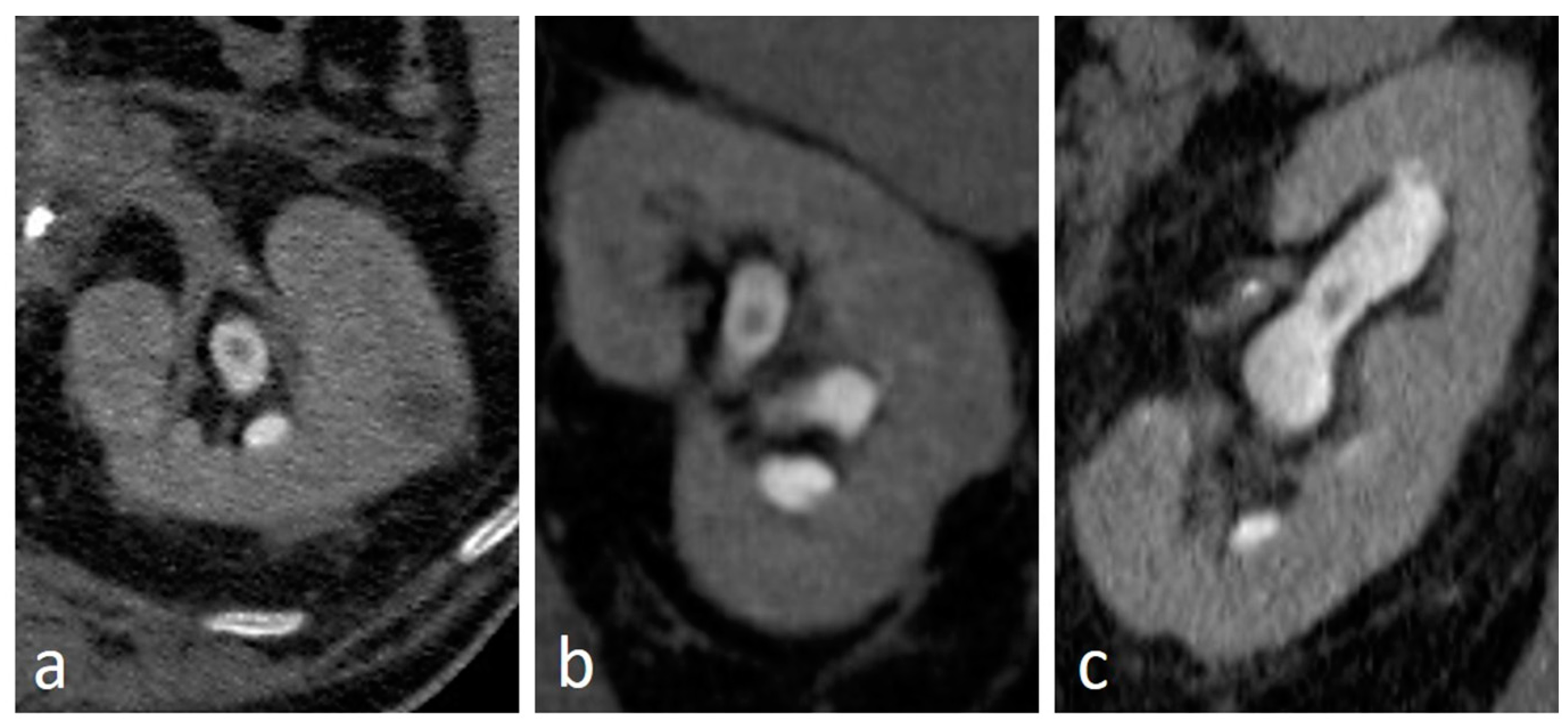
4.3. Suburothelial Hemorrhage
4.4. Renal Papillary Necrosis
4.5. Inflammation
4.6. Retroperitoneal Fibrosis
4.7. Pyeloureteritis Cystica
4.8. Urogenital Tuberculosis
4.9. Fibroepithelial Polyp
4.10. Renal Cell Carcinoma
4.11. Renal Lymphoma
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CTU | Computed Tomography Urography; |
| FP | fibroepithelial polyp; |
| MIP | maximum intensity projection; |
| MPR | multiplanar reconstruction; |
| MRU | magnetic resonance urography; |
| RCC | renal cell carcinoma; |
| RF | retroperitoneal fibrosis; |
| RNP | renal papillary necrosis; |
| UC | urothelial carcinoma; |
| UGTB | urogenital tuberculosis; |
| UTUC | upper tract urothelial carcinoma |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.-M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernández, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Mostafid, A.H. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2020, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Janisch, F.; Shariat, S.F.; Baltzer, P.; Fajkovic, H.; Kimura, S.; Iwata, T.; Korn, P.; Yang, L.; Glybochko, P.V.; Rink, M.; et al. Diagnostic performance of multidetector computed tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): A systematic review and meta-analysis. World J. Urol. 2019, 38, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Martingano, P.; Cavallaro, M.F.M.; Bertolotto, M.; Stacul, F.; Ukmar, M.; Cova, M.A. Magnetic resonance urography vs computed tomography urography in the evaluation of patients with haematuria. Radiol. Med. 2013, 118, 1184–1198. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, A.J.; Cowan, N.C.; Mueller-Lisse, U.G.; Nolte-Ernsting, C.C.A.; Takahashi, S.; Cohan, R.H. CT urography: Definition, indications and techniques. A guideline for clinical practice. Eur. Radiol. 2007, 18, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Potenta, S.E.; D’Agostino, R.; Sternberg, K.M.; Tatsumi, K.; Perusse, K. CT Urography for Evaluation of the Ureter. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2015, 35, 709–726. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Vrtiska, T.J.; Leroy, A.J.; Hartman, R.P.; McCollough, C.H.; King, B.F. CT Urography. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2004, 24, S35–S54. [Google Scholar] [CrossRef]
- Caoili, E.M.; Cohan, R.H. CT urography in evaluation of urothelial tumors of the kidney. Abdom. Radiol. 2016, 41, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Cassidy, F.; Aganovic, L.; Taddonio, M.; Vahdat, N. CT urography: How to optimize the technique. Abdom. Radiol. 2019, 44, 3786–3799. [Google Scholar] [CrossRef]
- Silverman, S.G.; Leyendecker, J.R.; Amis, E.S. What Is the Current Role of CT Urography and MR Urography in the Evaluation of the Urinary Tract? Radiology 2009, 250, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Shampain, K.L.; Cohan, R.H.; Caoili, E.M.; Davenport, M.S.; Ellis, J.H. Benign diseases of the urinary tract at CT and CT urography. Abdom. Radiol. 2019, 44, 3811–3826. [Google Scholar] [CrossRef] [PubMed]
- Martingano, P.; Stacul, F.; Cavallaro, M.F.; Cernic, S.; Bregant, P.; Cova, M.A. 64-Slice CT urography: Optimisation of radiation dose. Radiol. Med. 2011, 116, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Fishman, E.K.; Sheth, S. Upper urinary tract urothelial carcinoma on multidetector CT: Spectrum of disease. Abdom. Radiol. 2019, 44, 3874–3885. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.F.J.; Meehan, C.P.; Colville, J.; Power, R.; Torreggiani, W.C. Transitional Cell Carcinoma of the Upper Urinary Tract: Spectrum of Imaging Findings. Radiographics 2005, 25, 1609–1627. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, S.; Horton, K.M.; Fishman, E.K. Transitional Cell Neoplasm of the Upper Urinary Tract: Evaluation with MDCT. Am. J. Roentgenol. 2008, 191, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Dalla-Palma, L.; Mucelli, R.P. Malignant Renal Neoplasms. In Radiation Therapy of Benign Diseases; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1991; pp. 251–274. [Google Scholar]
- Prando, A.; Prando, P.; Prando, D. Urothelial Cancer of the Renal Pelvicaliceal System: Unusual Imaging Manifestations 1. Radiographics 2010, 30, 1553–1566. [Google Scholar] [CrossRef]
- Wong-You-Cheong, J.J.; Wagner, B.J.; Davis, C.J., Jr. Transitional Cell Carcinoma of the Urinary Tract: Radiologic-Pathologic Correlation. Radiographics 1998, 18, 123–142. [Google Scholar] [CrossRef]
- Dinsmore, B.J.; Pollack, H.M.; Banner, M.P. Calcified transitional cell carcinoma of the renal pelvis. Radiology 1988, 167, 401–404. [Google Scholar] [CrossRef]
- Quaia, E.; Martingano, P.; Cavallaro, M.; Premm, M.; Angileri, R. Normal Radiological Anatomy and Anatomical Variants of the Kidney. In Radiation Therapy of Benign Diseases; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; pp. 17–74. [Google Scholar]
- Dyer, R.B.; Chen, M.Y.; Zagoria, R.J. Classic Signs in Uroradiology. Radiographics 2004, 24, S247–S280. [Google Scholar] [CrossRef]
- Fritz, G.A.; Schoellnast, H.; Deutschmann, H.A.; Quehenberger, F.; Tillich, M. Multiphasic multidetector-row CT (MDCT) in detection and staging of transitional cell carcinomas of the upper urinary tract. Eur. Radiol. 2006, 16, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Cansever, A.T.; Erden, A.; Ölçer, T.; Cumhur, T. Hypertrophic renal papillae mimicking urothelial tumor. Eur. J. Radiol. Extra 2009, 69, e121–e123. [Google Scholar] [CrossRef]
- Kawamoto, S.; Duggan, P.; Sheth, S.; Miyamoto, H.; Kazi, Z.N.; Fishman, E.K. Renal Papillary and Calyceal Lesions at CT Urography: Genitourinary Imaging. Radiographics 2017, 37, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Gayer, G.; Desser, T.S.; Hertz, M.; Osadchy, A.; Daniel, B.L.; Zissin, R. Spontaneous Suburothelial Hemorrhage in Coagulopathic Patients: CT Diagnosis. Am. J. Roentgenol. 2011, 197, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Gumeler, E.; Onur, M.R.; Karaosmanoglu, A.D.; Ozmen, M.; Akata, D.; Karcaaltincaba, M. Computed tomography and magnetic resonance imaging of peripelvic and periureteric pathologies. Abdom. Radiol. 2017, 43, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Tang, G.; Hou, Z.; Wang, G. Transitional cell carcinoma of upper urinary tract vs. benign lesions: Distinctive MSCT features. Abdom. Imaging 2008, 34, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Wasnik, A.P.; Elsayes, K.M.; Kaza, R.K.; Al-Hawary, M.M.; Cohan, R.H.; Francis, I.R. Multimodality Imaging in Ureteric and Periureteric Pathologic Abnormalities. Am. J. Roentgenol. 2011, 197, W1083–W1092. [Google Scholar] [CrossRef]
- Noorbakhsh, A.; Aganovic, L.; Vahdat, N.; Fazeli, S.; Chung, R.; Cassidy, F.H. What a difference a delay makes! CT urogram: A pictorial essay. Abdom. Radiol. 2019, 44, 3919–3934. [Google Scholar] [CrossRef] [PubMed]
- Gaudiano, C.; Tadolini, M.; Busato, F.; Vanino, E.; Pucci, S.; Corcioni, B.; Golfieri, R. Multidetector CT urography in urogenital tuberculosis: Use of reformatted images for the assessment of the radiological findings. A pictorial essay. Abdom. Radiol. 2017, 42, 2314–2324. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Kim, J.K.; Cho, K.-S. Genitourinary Tuberculosis: Comprehensive Cross-Sectional Imaging. Am. J. Roentgenol. 2005, 184, 143–150. [Google Scholar] [CrossRef]
- Wong, A.; Dhingra, S.; Surabhi, V.R. AIRP Best Cases in Radiologic-Pathologic Correlation: Genitourinary Tuberculosis. Radiographics 2012, 32, 839–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vikram, R.; Sandler, C.M.; Ng, C.S. Imaging and Staging of Transitional Cell Carcinoma: Part 2, Upper Urinary Tract. Am. J. Roentgenol. 2009, 192, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Ali, S.; Fishman, E. Imaging of Renal Lymphoma: Patterns of Disease with Pathologic Correlation. Radiographics 2006, 26, 1151–1168. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, D.; Iyer, R.; Devine, C.; Bhosale, P.; Paulson, E. Imaging of Primary and Secondary Renal Lymphoma. Am. J. Roentgenol. 2013, 201, W712–W719. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martingano, P.; Cavallaro, M.F.M.; Bozzato, A.M.; Baratella, E.; Cova, M.A. CT Urography Findings of Upper Urinary Tract Carcinoma and Its Mimickers: A Pictorial Review. Medicina 2020, 56, 705. https://doi.org/10.3390/medicina56120705
Martingano P, Cavallaro MFM, Bozzato AM, Baratella E, Cova MA. CT Urography Findings of Upper Urinary Tract Carcinoma and Its Mimickers: A Pictorial Review. Medicina. 2020; 56(12):705. https://doi.org/10.3390/medicina56120705
Chicago/Turabian StyleMartingano, Paola, Marco F. M. Cavallaro, Alessandro M. Bozzato, Elisa Baratella, and Maria A. Cova. 2020. "CT Urography Findings of Upper Urinary Tract Carcinoma and Its Mimickers: A Pictorial Review" Medicina 56, no. 12: 705. https://doi.org/10.3390/medicina56120705
APA StyleMartingano, P., Cavallaro, M. F. M., Bozzato, A. M., Baratella, E., & Cova, M. A. (2020). CT Urography Findings of Upper Urinary Tract Carcinoma and Its Mimickers: A Pictorial Review. Medicina, 56(12), 705. https://doi.org/10.3390/medicina56120705




