Obstruction of the Hepatic Venous Flow Caused by Intravenous Leiomyomatosis
Abstract
1. Introduction
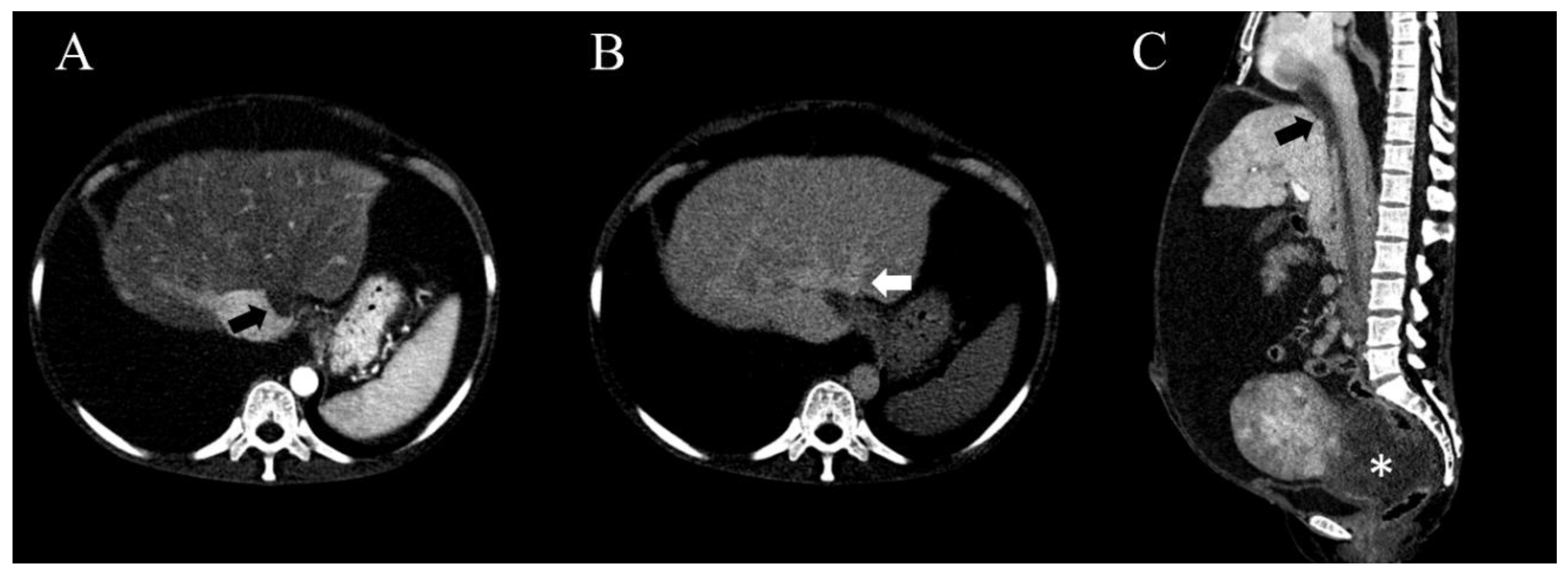
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Norris, H.J.; Parmley, T. Mesenchymal tumors of the uterus. V. Intravenous leiomyomatosis: A clinical and pathologic study of 14 cases. Cancer 1975, 36, 2164–2178. [Google Scholar] [CrossRef]
- Birch-Hirschfeld, F.V. Lehrbuch der Pathologischen Anatomie; Leipzig: F.C.W. Vogel: Leipzig, Germany, 1896. [Google Scholar]
- Shi, T.; Shkrum, M.J. A case report of sudden death from intracardiac leiomyomatosis. Am. J. Forensic. Med. Pathol. 2018, 39, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.C.; Boitnott, J.K.; Kaufman, S.; Cameron, J.L.; Maddrey, W.C. Budd-Chiari syndrome: Etiology, diagnosis and management. Medicine 1982, 61, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Hemming, A.W.; Langer, B.; Greig, P.; Taylor, B.R.; Adams, R.; Heathcote, E.J. Treatment of Budd-Chiari syndrome with portosystemic shunt or liver transplantation. Am. J. Surg. 1996, 171, 176–181. [Google Scholar] [CrossRef]
- Barksdale, J.; Abolhoda, A.; Saremi, F. Intravenous leiomyomatosis presenting as acute Budd-Chiari syndrome. J. Vasc. Surg. 2011, 54, 860–863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peh, W.C.; Cheung, D.L.; Ngan, H. Smooth muscle tumors of the inferior vena cava and right heart. Clin. Imaging 1993, 17, 117–123. [Google Scholar] [CrossRef]
- Kuenen, B.; Slee, P.; Seldenrijk, C.; Wagenaar, S. Intravenous leiomyomatosis complicated by Budd—Chiari syndrome. Postgrad. Med. J. 1996, 72, 686. [Google Scholar] [CrossRef]
- Hadengue, A.; Poliquin, M.; Vilgrain, V.; Belghiti, J.; Degott, C.; Erlinger, S.; Benhamou, J.-P. The changing scene of hepatic vein thrombosis: Recognition of asymptomatic cases. Gastroenterology 1994, 106, 1042–1047. [Google Scholar] [CrossRef]
- Langlet, P.; Escolano, S.; Valla, D.; Coste-Zeitoun, D.; Denie, C.; Mallet, A.; Levy, V.-G.; Franco, D.; Vinel, J.-P.; Belghiti, J.; et al. Clinicopathological forms and prognostic index in Budd-Chiari syndrome. J. Hepatol. 2003, 39, 496–501. [Google Scholar] [CrossRef]
- Menon, K.N.; Shah, V.; Kamath, P.S. The Budd–Chiari syndrome. N. Engl. J. Med. 2004, 350, 578–585. [Google Scholar] [CrossRef]
- Liu, N.; Long, Y.; Liu, Y. Intravenous leiomyomatosis: Case series and review of the literature. J. Int. Med. Res. 2020, 48, 0300060519896887. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.-Q.; Zhang, B.; Liu, B.-G.; Liu, F.-H. Diagnosis of intravenous leiomyomatosis extending to heart with emphasis on magnetic resonance imaging. Chin. Med. J. 2012, 125, 33–37. [Google Scholar] [PubMed]
- de Franchis, R. Evolving Consensus in Portal Hypertension Report of the Baveno IV Consensus Workshop on methodology of diagnosis and therapy in portal hypertension. J. Hepatol. 2005, 43, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Ren, W.; Wang, Y.; Guo, X.; Fan, D. Survival and prognostic indicators of Budd–Chiari syndrome: A systematic review of 79 studies. Expert. Rev. Gastroenterol. Hepatol. 2015, 9, 865–875. [Google Scholar] [CrossRef]
- Virzì, G.; Ragazzi, S.; Bussichella, F.; D’Agati, P.; Caputo, S.; Scaravilli, F.; Piazza, D. Intravenous leiomyomatosis extending from the inferior caval vein to the pulmonary artery. J. Thorac. Cardiovasc. Surg. 2007, 133, 831–832. [Google Scholar] [CrossRef][Green Version]
- Ordulu, Z.; Nucci, M.R.; Dal Cin, P.; Hollowell, M.L.; Otis, C.N.; Hornick, J.L.; Park, P.J.; Kim, T.-M.; Quade, B.J.; Morton, C.C. Intravenous leiomyomatosis: An unusual intermediate between benign and malignant uterine smooth muscle tumors. Mod. Pathol. 2016, 29, 500–510. [Google Scholar] [CrossRef]
- Lam, P.M.; Lo, K.W.; Mei, Y.Y.; Wong, W.S.; Lau, J.Y.; Arifi, A.A.; Cheung, T.H. Intravenous leiomyomatosis: Two cases with different routes of tumor extension. J. Vasc. Surg. 2004, 39, 465–469. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, X.; Shi, H.; Fan, Q.; Lang, J.; Liu, B. Clinical characteristics and prognostic features of intravenous leiomyomatosis with inferior vena cava or intracardiac extension. J Vasc Surg Venous Lymphat Disord. 2017, 5, 485–492. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Tavakolli, A.; Safaei, A. Recurrent intracardiac leiomyomatosis. Can. J. Cardiol. 2007, 23, 1085–1086. [Google Scholar] [CrossRef][Green Version]
- Liu, B.; Liu, C.; Guan, H.; Li, Y.; Song, X.; Shen, K.; Miao, Q. Intravenous leiomyomatosis with inferior vena cava and heart extension. J. Vasc. Surg. 2009, 50, 897–902. [Google Scholar] [CrossRef]
- DeRubertis, B.G.; Clair, D.; Faries, P.; Kapur, S.; Park, K.; Kent, K.C. Resection of an intravenous leiomyoma with intracardiac extension with use of endovascular techniques. J. Vasc. Surg. 2004, 40, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Al-Hendy, A. Innovative oral treatments of uterine leiomyoma. Obstet. Gynecol. Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Wiltshaw, E.; Kochanowski, S.; Macfarlane, A.; Sears, R. Metastasizing leiomyoma of the uterus and hormonal manipulations. Case report. BJOG Int. J. Obstet. Gynaecol. 1986, 93, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.-K.; Lau, T.-K. Intracardiac leiomyomatosis. Arch. Gynecol. Obstet. 2001, 264, 209–210. [Google Scholar] [CrossRef]
- Solomon, L.A.; Schimp, V.L.; Ali-Fehmi, R.; Diamond, M.P.; Munkarah, A.R. Clinical update of smooth muscle tumors of the uterus. J. Minim. Invasive. Gynecol. 2005, 12, 401–408. [Google Scholar] [CrossRef]
- Kir, G.; Kir, M.; Gurbuz, A.; Karateke, A.; Aker, F. Estrogen and progesterone expression of vessel walls with intravascular leiomyomatosis; discussion of histogenesis. Eur. J. Gynaecol. Oncol. 2004, 25, 362–366. [Google Scholar]
- Barjot, P.J.; Refahi, N.; Berthet, P.; Delautre, V.D. Intravenous leiomyomatosis of the uterus: A GnRH agonist utilisation before surgery. J. Obstet. Gynaecol. 1998, 18, 492–493. [Google Scholar]
- Mitsuhashi, A.; Nagai, Y.; Sugita, M.; Nakajima, N.; Sekiya, S. GnRH agonist for intravenous leiomyomatosis with cardiac extension. A case report. J. Reprod. Med. 1999, 44, 883–886. [Google Scholar]
- Khayata, G.; Thwaini, S.; Aswad, S. Intravenous leiomyomatosis extending to the heart. Int. J. Gynaecol. Obstet. 2003, 80, 59–60. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Yeo, I.H.; Kim, Y.J.; Kim, J.K. Obstruction of the Hepatic Venous Flow Caused by Intravenous Leiomyomatosis. Medicina 2020, 56, 696. https://doi.org/10.3390/medicina56120696
Park S-Y, Yeo IH, Kim YJ, Kim JK. Obstruction of the Hepatic Venous Flow Caused by Intravenous Leiomyomatosis. Medicina. 2020; 56(12):696. https://doi.org/10.3390/medicina56120696
Chicago/Turabian StylePark, Sin-Youl, In Hwan Yeo, Yun Jeong Kim, and Jong Kun Kim. 2020. "Obstruction of the Hepatic Venous Flow Caused by Intravenous Leiomyomatosis" Medicina 56, no. 12: 696. https://doi.org/10.3390/medicina56120696
APA StylePark, S.-Y., Yeo, I. H., Kim, Y. J., & Kim, J. K. (2020). Obstruction of the Hepatic Venous Flow Caused by Intravenous Leiomyomatosis. Medicina, 56(12), 696. https://doi.org/10.3390/medicina56120696




