Anti-Osteoarthritic Mechanisms of Chrysanthemum zawadskii var. latilobum in MIA-Induced Osteoarthritic Rats and Interleukin-1β-Induced SW1353 Human Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CZE
2.2. High-Performance Liquid Chromatography Analysis of CZE
2.3. Animals
2.4. Induction of OA and the In Vivo Experimental Design
2.5. Staining for Histological Analysis
2.6. Microarray Analysis
2.7. Human Chondrosarcoma Cell Line SW1353 Culture for In Vitro Experiment
2.8. Induction of OA in Cell Culture and the In Vitro Experimental Design
2.9. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
2.10. Western Blot Assay
2.11. Statistical Analysis
3. Results
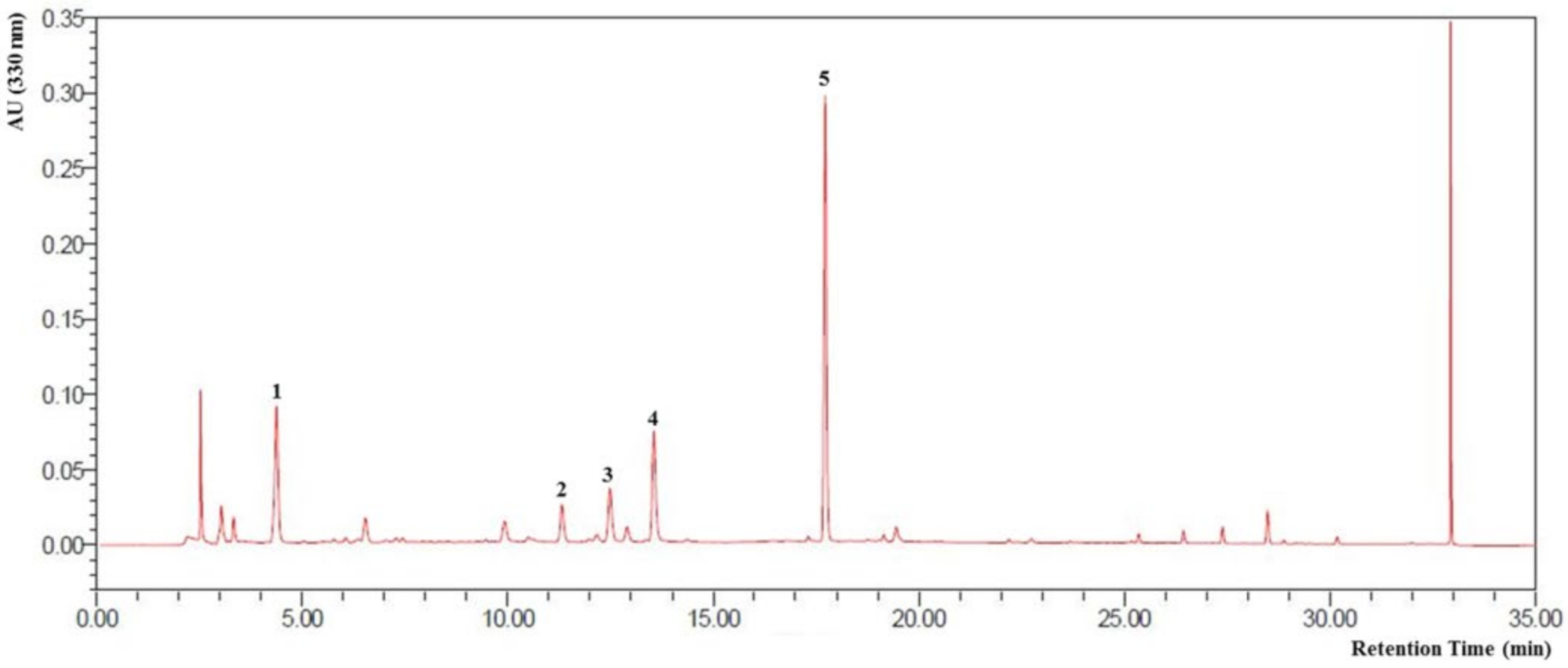
3.1. HPLC Profile of CZE and Verification of Its Components
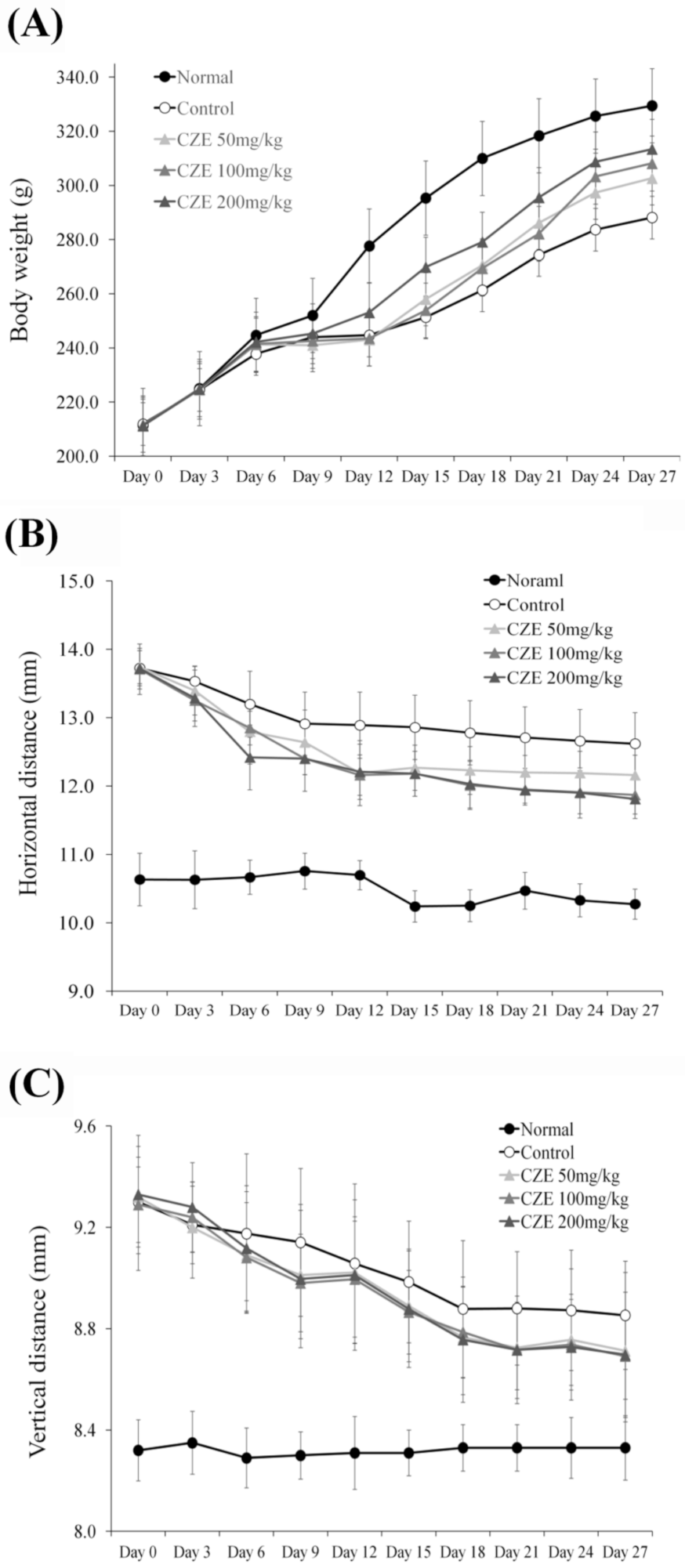
3.2. CZE Reversed Weight Loss in MIA-Induced OA Rats
3.3. CZE Improved Articular Edema in MIA-Induced OA Rats
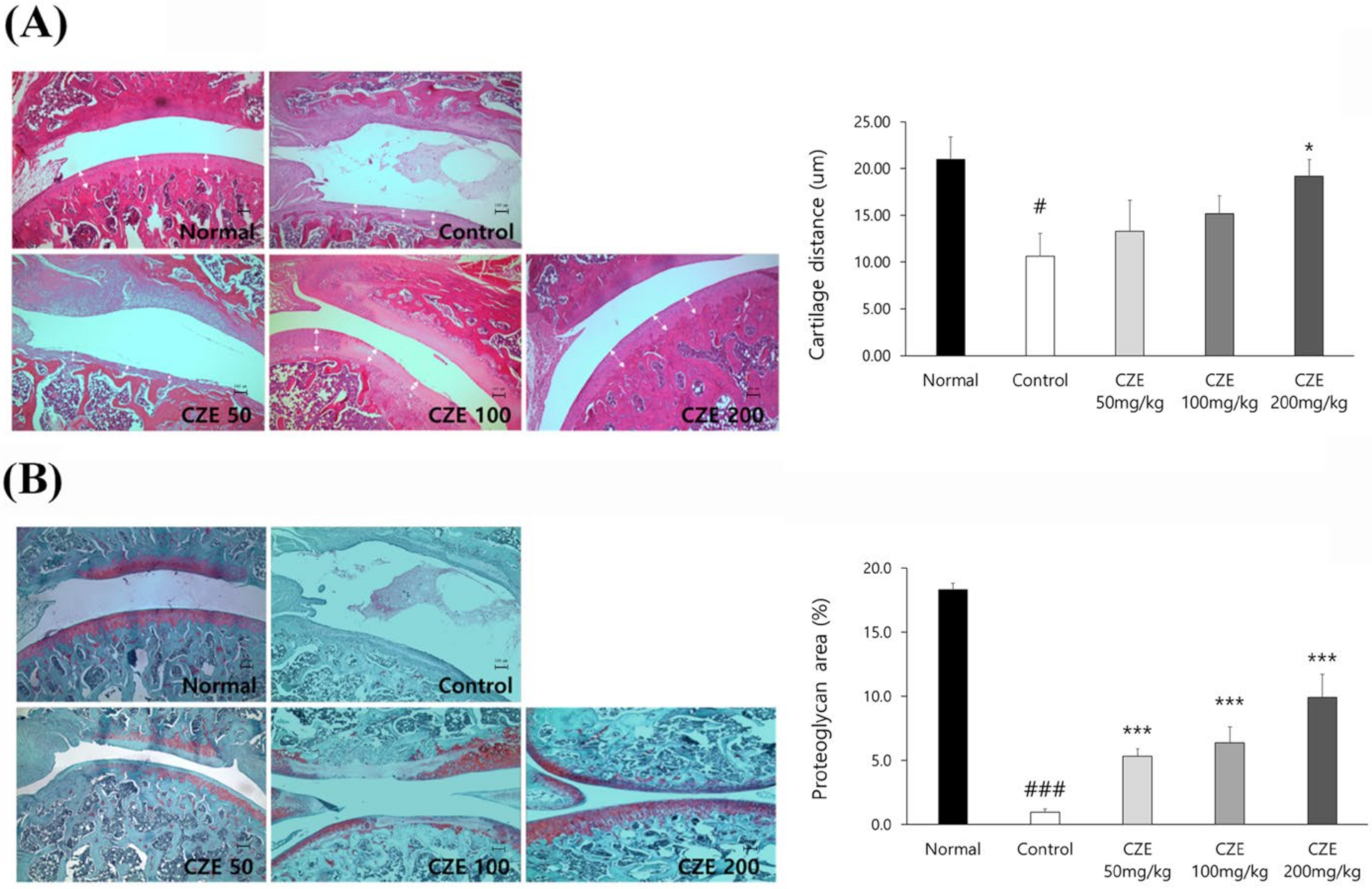
3.4. CZE Improved the Articular Structure in Mia-Induced OA Rats
3.5. Pro-Inflammatory and ECM-Degrading Marker Levels Were Increased in the Articular Cartilage of MIA-Induced OA Rats
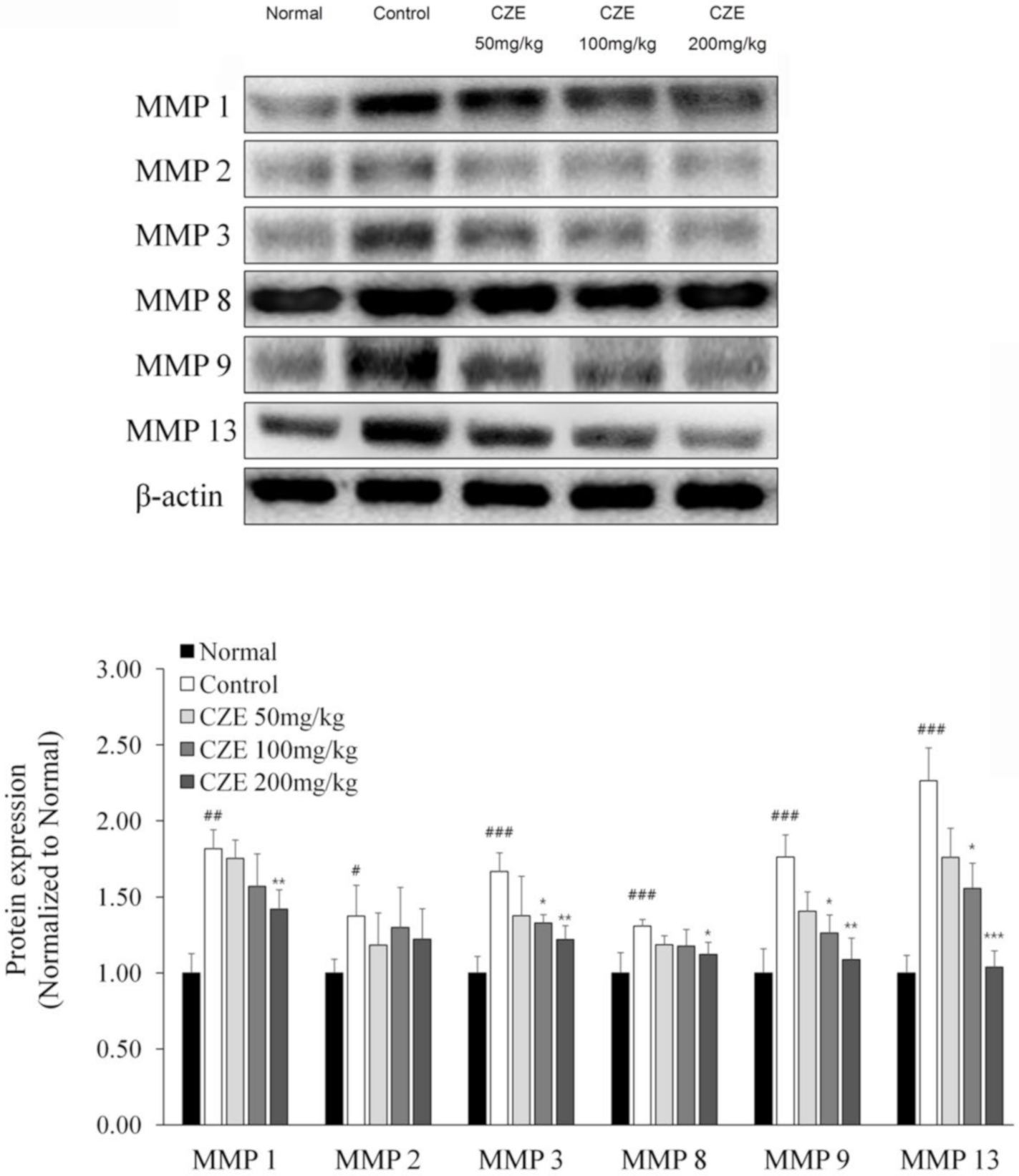
3.6. CZE Decreased the Levels of Six MMPs in Articular Cartilage of MIA-Induced OA Rats
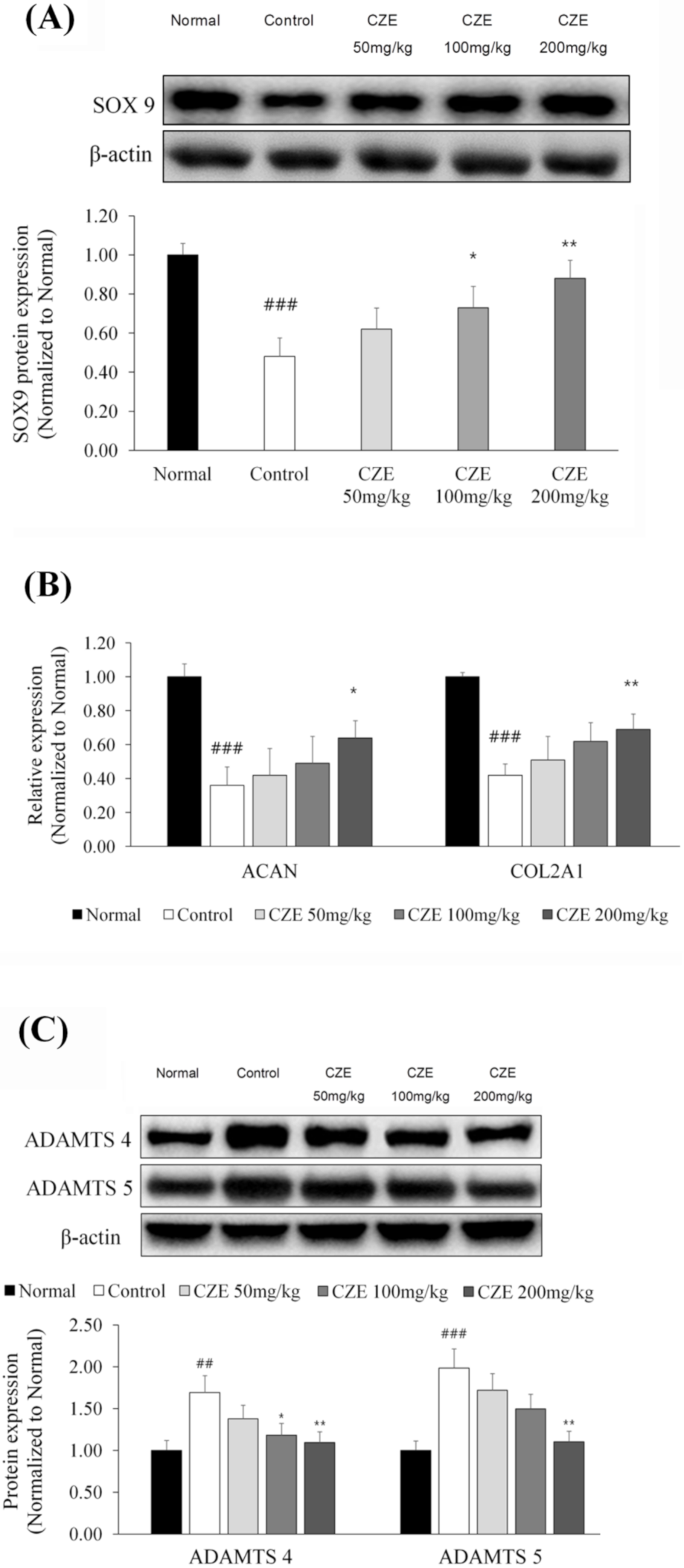
3.7. CZE Increased the Expression of SOX9 and ECM Synthetic Genes in Articular Cartilage of MIA-Induced OA Rats
3.8. CZE Decreased the Levels of Two ADAMTSs in Articular Cartilage of MIA-Induced OA Rats
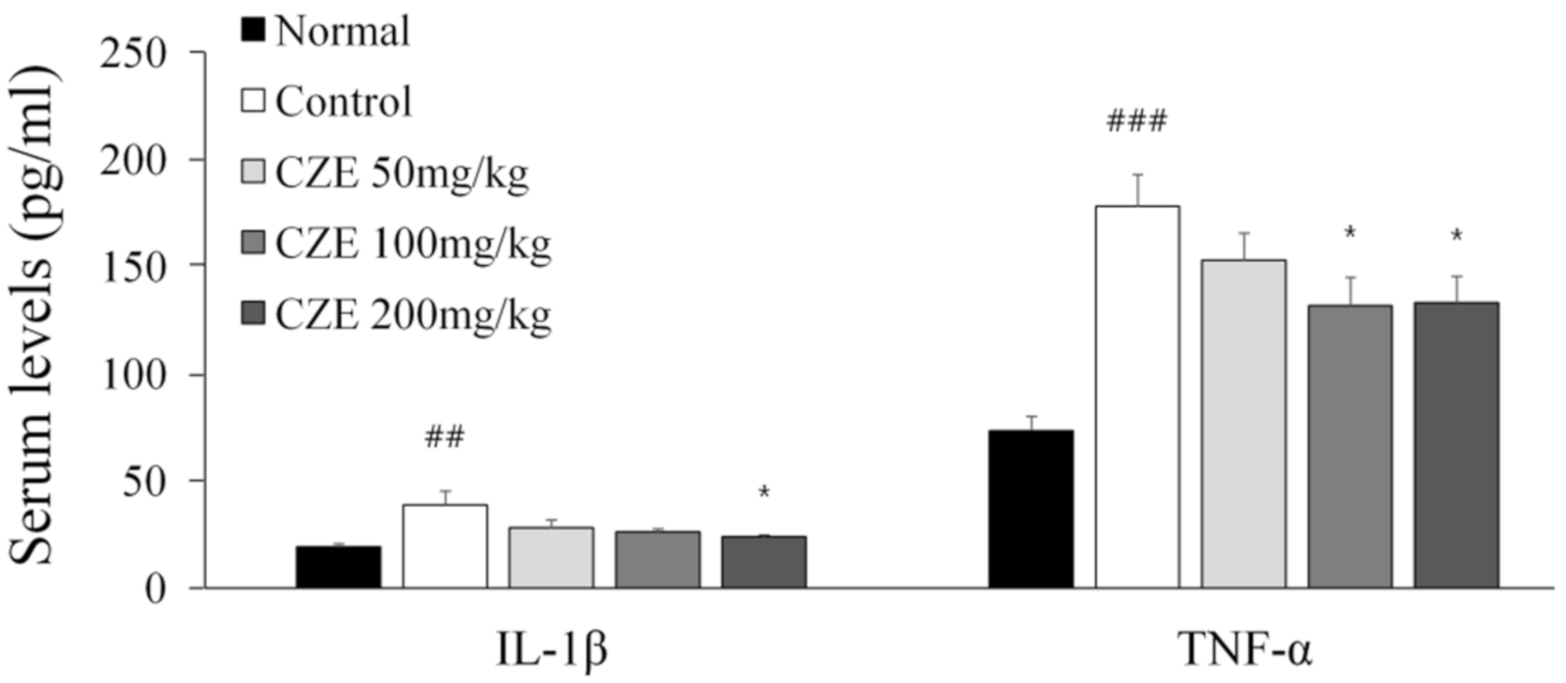
3.9. CZE Decreased Serum IL-1β and TNF-α Levels in MIA-Induced OA Rats
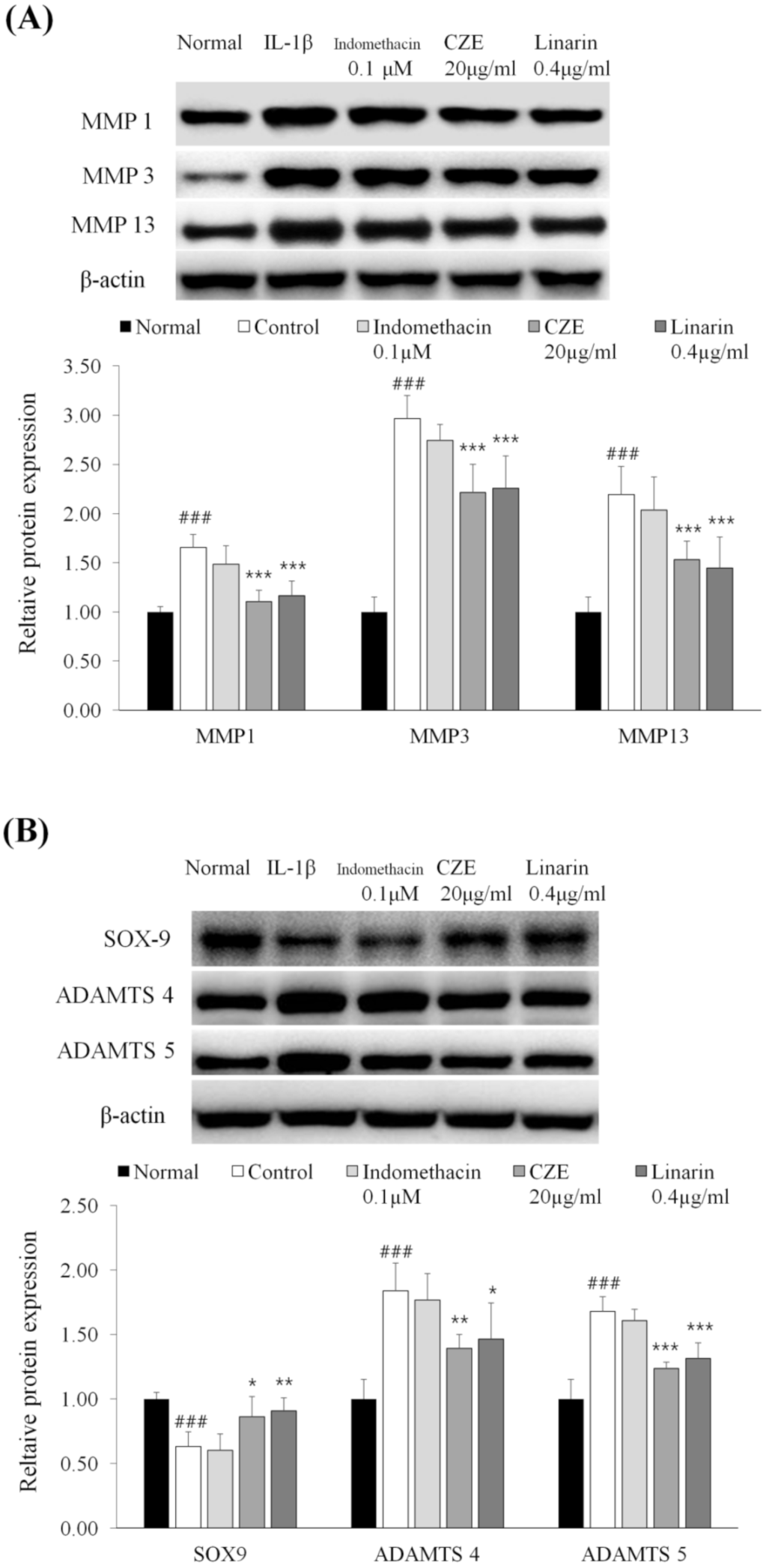
3.10. Linarin, the Marker Compound of CZE, Decreased the Levels of MMPs in IL-1β-Treated Human Chondrosarcoma Cell Line SW1353
3.11. Linarin Increased the Expression of SOX9 and Decreased the Levels of Two ADAMTSs in IL-1β-Treated Human Chondrosarcoma Cell Line SW1353
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease, Professional Edition; Elsevier Health Sciences: London, UK, 2014; p. 1208. Available online: https://www.pdfdrive.com/robbins-and-cotran-pathologic-basis-of-disease-professional-edition-e185978689.html (accessed on 9 November 2020).
- Chen, C.; Xie, J.; Rajappa, R.; Deng, L.; Fredberg, J.; Yang, L. Interleukin-1β and tumor necrosis factor-α increase stiffness and impair contractile function of articular chondrocytes. Acta Biochim. Biophys. Sin. 2014, 47, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Ellman, M.B.; Yan, D.; Kroin, J.S.; Cole, B.J.; Van Wijnen, A.J.; Im, H.-J. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 2013, 527, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Taskiran, D.; Stefanovic Racic, M.; Georgescu, H.; Evans, C. Nitric-Oxide Mediates Suppression of Cartilage Proteoglycan Synthesis by Interleukin-1. Biochem. Biophys. Res. Commun. 1994, 200, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Nagase, H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: Destruction or repair? Nat. Clin. Pr. Rheumatol. 2008, 4, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jotanovic, Z.; Mihelic, R.; Sestan, B.; Dembic, Z. Role of Interleukin-1 Inhibitors in Osteoarthritis. Drugs Aging 2012, 29, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sung, K.-H.; Yim, D.; Lee, S.; Lee, C.-K.; Ha, N.-J.; Kim, K. The effect of linarin on lps-lnduced cytokine production and nitric oxide inhibition in murine macrophages cell line RAW264.7. Arch. Pharmacal Res. 2002, 25, 170–177. [Google Scholar] [CrossRef]
- Kim, Y.; Han, J.; Sung, J.; Sung, M.; Choi, Y.; Jeong, H.S.; Lee, J. Anti-inflammatory activity of Chrysanthemum zawadskii var. latilobum leaf extract through haem oxygenase-1 induction. J. Funct. Foods 2012, 4, 474–479. [Google Scholar] [CrossRef]
- Kim, A.-R.; Kim, H.S.; Kim, D.K.; Lee, J.H.; Yoo, Y.H.; Kim, J.Y.; Park, S.K.; Nam, S.T.; Kim, H.W.; Park, Y.H.; et al. The Extract of Chrysanthemum zawadskii var. latilobum Ameliorates Collagen-Induced Arthritis in Mice. Evid. Based Complement. Altern. Med. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Hong, J.-M.; Shin, J.-K.; Kim, J.-Y.; Jang, M.-J.; Park, S.-K.; Lee, J.-H.; Choi, J.-H.; Lee, S.-M. BST106 Protects against Cartilage Damage by Inhibition of Apoptosis and Enhancement of Autophagy in Osteoarthritic Rats. Biol. Pharm. Bull. 2018, 41, 1257–1268. [Google Scholar] [CrossRef]
- Gebauer, M.; Saas, J.; Sohler, F.; Haag, J.; Söder, S.; Pieper, M.; Bartnik, E.; Beninga, J.; Zimmer, R.; Aigner, T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1β. Osteoarthr. Cartil. 2005, 13, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Cho, H.-I.; Kim, S.-J.; Park, J.-H.; Kim, J.-S.; Kim, Y.H.; Lee, S.K.; Kwak, J.-H.; Lee, S.-M. Protective effect of linarin against d-galactosamine and lipopolysaccharide-induced fulminant hepatic failure. Eur. J. Pharmacol. 2014, 738, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.P.; Liang, S.; Akdemir, K.C.; De Crombrugghe, B. The postnatal role of Sox9 in cartilage. J. Bone Miner. Res. 2012, 27, 2511–2525. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Okano, H.; Miyagawa, W.; Visse, R.; Shitomi, Y.; Santamaria, S.; Dudhia, J.; Troeberg, L.; Strickland, D.K.; Hirohata, S.; et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016, 56, 57–73. [Google Scholar] [CrossRef]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Nagao, M.; Hamilton, J.L.; Kc, R.; Berendsen, A.D.; Duan, X.; Cheong, C.W.; Li, X.; Im, H.-J.; Olsen, B.R. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Grumati, P.; Dikic, I. Ubiquitin signaling and autophagy. J. Biol. Chem. 2018, 293, 5404–5413. [Google Scholar] [CrossRef]
- Jeon, H.; Im, G.-I. Autophagy in osteoarthritis. Connect. Tissue Res. 2016, 58, 497–508. [Google Scholar] [CrossRef]
- Li, Y.-S.; Zhang, F.-J.; Zeng, C.; Luo, W.; Xiao, W.-F.; Gao, S.-G.; Lei, G.-H. Autophagy in osteoarthritis. Jt. Bone Spine 2016, 83, 143–148. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, L.; Hsieh, M.-S.; Chang, C.-P.; Chou, D.-T.; Tsai, S.-H. Evidence for a protective role for adiponectin in osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 711–718. [Google Scholar] [CrossRef]
- Lai, Q.; Liu, Y.; Huang, L.; Liu, X.; Yu, X.; Wang, Q.; Guo, R.; Zhu, J.; Cheng, H.; Dai, M.; et al. Expression of adiponectin in the subchondral bone of lumbar facet joints with different degrees of degeneration. BMC Musculoskelet. Disord. 2017, 18, 427. [Google Scholar] [CrossRef] [PubMed]
- Coskun, N.C.; Ay, S.; Evcik, F.D.; Oztuna, D. Adiponectin: Is it a biomarker for assessing the disease severity in knee osteoarthritis patients? Int. J. Rheum. Dis. 2015, 20, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Hu, Z.-C.; Shen, L.-Y.; Shang, P.; Xu, H.-Z.; Liu, H.-X. Association of osteoarthritis and circulating adiponectin levels: A systematic review and meta-analysis. Lipids Health Dis. 2018, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.; Aspenberg, P. Metalloproteinases and their inhibitors—Diagnostic and therapeutic opportunities in orthopedics. Acta Orthop. 2009, 80, 693–703. [Google Scholar] [CrossRef]
- Bauvois, B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim. Biophys. Acta Rev. Cancer 2012, 1825, 29–36. [Google Scholar] [CrossRef]
- Little, C.; Barai, A.; Burkhardt, D.; Smith, S.M.; Fosang, A.J.; Werb, Z.; Shah, M.H.; Thompson, E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009, 60, 3723–3733. [Google Scholar] [CrossRef]
- Neuhold, L.A.; Killar, L.; Zhao, W.; Sung, M.-L.A.; Warner, L.; Kulik, J.; Turner, J.; Wu, W.; Billinghurst, C.; Meijers, T.; et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Investig. 2001, 107, 35–44. [Google Scholar] [CrossRef]
- Galasso, O.; Familiari, F.; De Gori, M.; Gasparini, G. Recent Findings on the Role of Gelatinases (Matrix Metalloproteinase-2 and -9) in Osteoarthritis. Adv. Orthop. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, J.; He, Q.; Wang, Z. Cartilage oligomeric matrix protein, C-terminal cross-linking telopeptide of type II collagen, and matrix metalloproteinase-3 as biomarkers for knee and hip osteoarthritis (OA) diagnosis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2019, 27, 726–736. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, A.; Li, W.; Song, J.; Gao, C. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef]
- Chang, J.J.; Stanfill, A.G.; Pourmotabbed, T. The Role of Matrix Metalloproteinase Polymorphisms in Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 1323. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dancevic, C.M.; McCulloch, D.R. Current and emerging therapeutic strategies for preventing inflammation and aggrecanase-mediated cartilage destruction in arthritis. Arthritis Res. Ther. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stanton, H.; Rogerson, F.M.; East, C.J.; Golub, S.B.; Lawlor, K.E.; Meeker, C.T.; Little, C.B.; Last, K.; Farmer, P.J.; Campbell, I.K.; et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 2005, 434, 648–652. [Google Scholar] [CrossRef]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.; Blanchet, T.; Ma, H.-L.; Flannery, C.R.; Peluso, D.; Kanki, K.; Yang, Z.; et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nat. Cell Biol. 2005, 434, 644–648. [Google Scholar] [CrossRef]
- Malfait, A.-M.; Liu, R.-Q.; Ijiri, K.; Komiya, S.; Tortorella, M.D. Inhibition of ADAM-TS4 and ADAM-TS5 Prevents Aggrecan Degradation in Osteoarthritic Cartilage. J. Biol. Chem. 2002, 277, 22201–22208. [Google Scholar] [CrossRef]
- Zhang, E.; Yan, X.; Zhang, M.; Chang, X.; Bai, Z.; He, Y.; Yuan, Z. Aggrecanases in the human synovial fluid at different stages of osteoarthritis. Clin. Rheumatol. 2013, 32, 797–803. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, Q.; Wang, X.; Kang, L.; Fu, Y.; Yin, Y.; Li, Z.; Liu, Y.; Xu, X.; Wang, Y. SOX9 is a regulator of ADAMTSs-induced cartilage degeneration at the early stage of human osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2259–2268. [Google Scholar] [CrossRef]
- Dingle, J. The effect of nonsteroidal antiinflammatory drugs on human articular cartilage glycosaminoglycan synthesis. Osteoarthr. Cartil. 1999, 7, 313–314. [Google Scholar] [CrossRef]
| Group 1: Pro-inflammatory Factors | Fold Change | ||||
|---|---|---|---|---|---|
| Gene Name | Full Protein Name | Antibody Name | C/N | S/N | S/C |
| Vcam1 | Vascular cell adhesion molecule 1 | CD106 | 1.657 | 1.380 | 0.833 |
| Fadd | Fas-associated protein with death domain | FADD | 1.579 | 1.190 | 0.753 |
| Csf2 | Granulocyte-macrophage colony-stimulating factor | GM-CSF | 1.649 | 1.356 | 0.822 |
| Ifng | Interferon-gamma | IFN-gamma | 1.305 | 1.135 | 0.870 |
| Il1a | Interleukin-1 alpha | IL-1 alpha | 1.261 | 1.047 | 0.830 |
| Il1b | Interleukin-1 beta | IL-1 beta | 1.568 | 1.073 | 0.684 |
| Il3 | Interleukin-3 | IL-3 | 1.253 | 1.205 | 0.962 |
| Il4 | Interleukin-4 | IL-4 | 1.661 | 1.299 | 0.782 |
| Il5 | Interleukin-5 | IL-5 | 1.453 | 1.112 | 0.765 |
| Il10 | Interleukin-10 | IL-10 | 1.553 | 1.195 | 0.769 |
| Il13 | Interleukin-13 | IL-13 | 1.779 | 1.299 | 0.730 |
| Cxcl10 | Interferon gamma-induced protein 10 | IP-10 | 1.572 | 1.141 | 0.726 |
| Ccl2 | Monocyte chemoattractant protein 1 | MCP-1 | 1.744 | 1.292 | 0.740 |
| Mif | Macrophage migration inhibitory factor | MIF | 1.337 | 1.060 | 0.793 |
| Ccl3 | Macrophage inflammatory protein 1-alpha | MIP-1 alpha | 1.295 | 1.327 | 1.025 |
| Cxcl2 | Macrophage inflammatory protein 2-alpha | MIP-2 alpha | 1.363 | 1.091 | 0.800 |
| Ccl20 | Macrophage inflammatory protein 3-alpha | MIP-3 alpha | 1.362 | 1.044 | 0.766 |
| Tgfb1 | Transforming growth factor beta 1 | TGF-beta1 | 1.216 | 0.960 | 0.789 |
| Tgfb3 | Transforming growth factor beta 3 | TGF-beta3 | 1.039 | 0.735 | 0.707 |
| Tnf | Tumor necrosis factor alpha | TNF-alpha | 1.482 | 1.164 | 0.785 |
| Tnfsf10 | TNF-related apoptosis-inducing ligand | TRAIL | 1.531 | 1.240 | 0.810 |
| Tnfrsf19 | TNF receptor superfamily, member 19 | TROY | 1.443 | 1.470 | 1.019 |
| Group 2: MMPs | Fold Change | ||||
| Gene Name | N | Antibody Name | C/N | S/N | S/C |
| Mmp2 | Matrix metalloproteinase-2 (gelatinase A) | MMP-2 | 1.202 | 1.269 | 1.056 |
| Mmp8 | Matrix metalloproteinase-8 | MMP-8 | 1.071 | 0.735 | 0.686 |
| Mmp13 | Matrix metalloproteinase-13 | MMP-13 | 1.409 | 0.732 | 0.520 |
| Group 3: Other Factors | Fold Change | ||||
| Gene Name | Protein Full Name | Antibody Name | C/N | S/N | S/C |
| Egfr | Epidermal growth factor receptor | EGFR | 1.521 | 1.128 | 0.741 |
| Nrp2 | Neuropilin 2 | Neuropilin-2 | 1.868 | 1.022 | 0.547 |
| Prlr | Prolactin receptor | Prolactin R | 1.276 | 0.994 | 0.779 |
| Vegfa | Vascular endothelial growth factor | VEGF | 1.506 | 1.183 | 0.786 |
| Adipoq | Adiponectin | 0.934 | 1.788 | 1.914 | |
| Ide | Insulin degrading enzyme | IDE | 0.937 | 1.456 | 1.554 |
| Retnlg | Resistin-like molecule | RELM beta | 0.695 | 1.088 | 1.566 |
| Retn | Resistin | 1.262 | 0.928 | 0.736 | |
| Ubiquitin | Ubiquitin | 0.697 | 1.854 | 2.659 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, J.-H.; Choi, C.-W.; Jang, M.-J.; Lim, S.H.; Han, H.J.; Choung, S.-Y. Anti-Osteoarthritic Mechanisms of Chrysanthemum zawadskii var. latilobum in MIA-Induced Osteoarthritic Rats and Interleukin-1β-Induced SW1353 Human Chondrocytes. Medicina 2020, 56, 685. https://doi.org/10.3390/medicina56120685
Byun J-H, Choi C-W, Jang M-J, Lim SH, Han HJ, Choung S-Y. Anti-Osteoarthritic Mechanisms of Chrysanthemum zawadskii var. latilobum in MIA-Induced Osteoarthritic Rats and Interleukin-1β-Induced SW1353 Human Chondrocytes. Medicina. 2020; 56(12):685. https://doi.org/10.3390/medicina56120685
Chicago/Turabian StyleByun, Jae-Hyuk, Chi-Won Choi, Min-Jung Jang, Su Hwan Lim, Hae Jung Han, and Se-Young Choung. 2020. "Anti-Osteoarthritic Mechanisms of Chrysanthemum zawadskii var. latilobum in MIA-Induced Osteoarthritic Rats and Interleukin-1β-Induced SW1353 Human Chondrocytes" Medicina 56, no. 12: 685. https://doi.org/10.3390/medicina56120685
APA StyleByun, J.-H., Choi, C.-W., Jang, M.-J., Lim, S. H., Han, H. J., & Choung, S.-Y. (2020). Anti-Osteoarthritic Mechanisms of Chrysanthemum zawadskii var. latilobum in MIA-Induced Osteoarthritic Rats and Interleukin-1β-Induced SW1353 Human Chondrocytes. Medicina, 56(12), 685. https://doi.org/10.3390/medicina56120685




