Primary Melanoma of the Lung: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
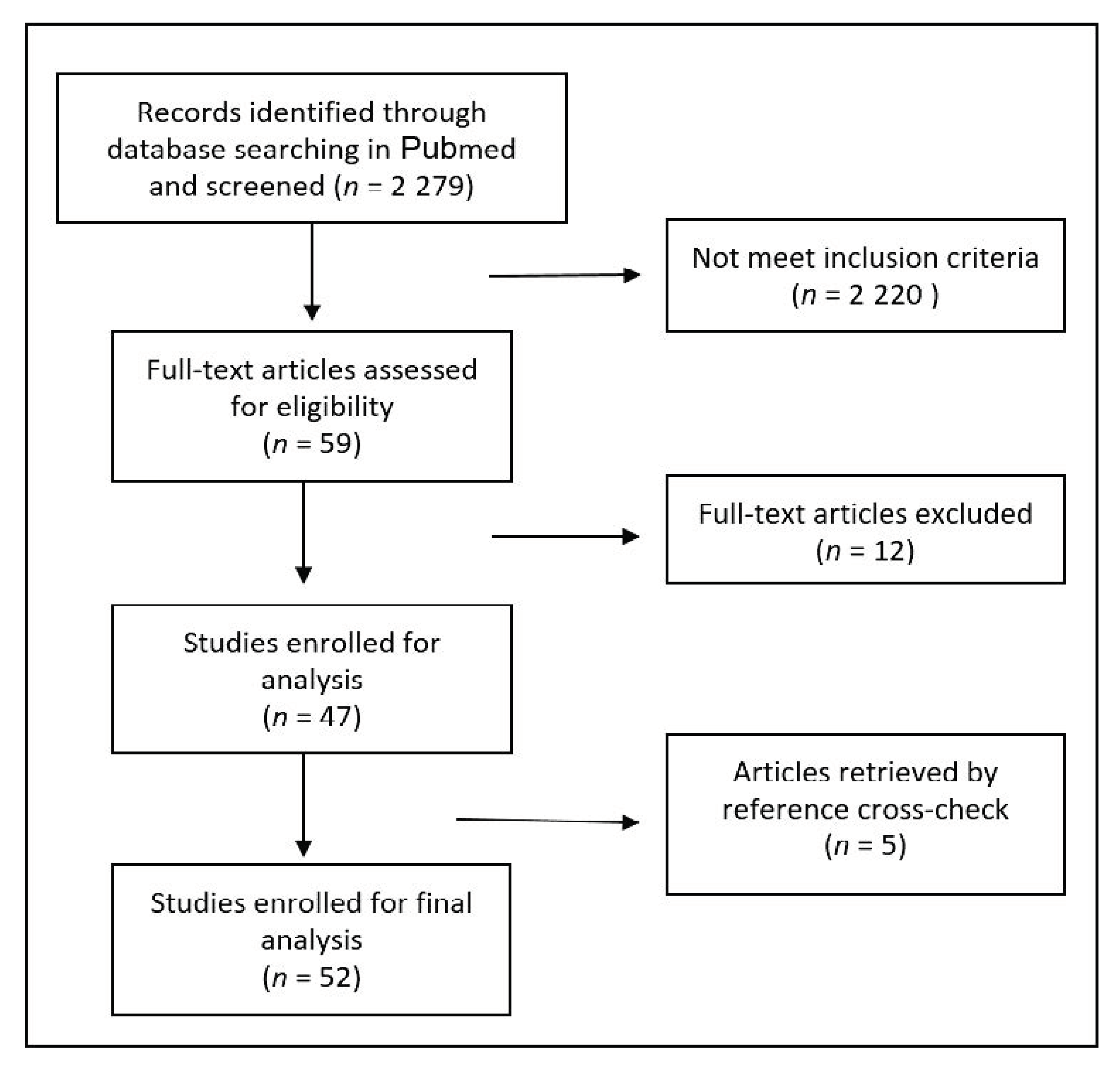
2.1. Search Strategy, Eligibility Criteria and Study Selection
2.2. Statistical Analysis and Reporting
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cossu, A.; Casula, M.; Cesaraccio, R.; Lissia, A.; Colombino, M.; Sini, M.C.; Budroni, M.; Tanda, F.; Paliogiannis, P.; Palmieri, G. Epidemiology and genetic susceptibility of malignant melanoma in North Sardinia, Italy. Eur. J. Cancer Prev. 2017, 26, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Attene, F.; Cossu, A.; Budroni, M.; Cesaraccio, R.; Tanda, F.; Trignano, M.; Palmieri, G. Lung cancer epidemiology in North Sardinia, Italy. Multidiscip. Respir. Med. 2013, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Budroni, M.; Cossu, A.; Paliogiannis, P.; Palmieri, G.; Attene, F.; Cesaraccio, R.; Tanda, F. Epidemiology of malignant pleural mesothelioma in the province of Sassari (Sardinia, Italy). A population-based report. Ann. Ital. Chir. 2013, 85, 244–248. [Google Scholar]
- Rodriguez, F.F.; Uddin, A.; Nasr, J. Primary Pulmonary Malignant Melanoma Found While Evaluating New Onset Cough: A Case Presentation and Literature Review. Case Rep. Pulmonol. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Little, J.; Rajkumar, C.; Saleem, W. A rare case of malignant meningitis from a likely bronchogenic melanoma primary cancer. Oxf. Med. Case Rep. 2019, 2019, omy114. [Google Scholar] [CrossRef]
- Parihar, A.S.; Ga, A.; Sood, K.; Seam, R.K.; Kaushal, V.; Sood, A.; Kakkar, N.; Mittal, B.R. Incidental Detection of Synchronous Lung Melanoma on 18F-FDG PET/CT in a Patient with Parotid Gland Myoepithelial Carcinoma. Clin. Nucl. Med. 2018, 43, e127–e129. [Google Scholar] [CrossRef] [PubMed]
- Yunce, M.; Selinger, S.; Krimsky, W.; Harley, D.P. Primary malignant melanoma of the lung: A case report of a rare tumor and review of the literature. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 29–31. [Google Scholar] [CrossRef]
- Yabuki, H.; Kuwana, K.; Minowa, M. Resection of primary malignant lung melanoma: A case report. Asian Cardiovasc. Thorac. Ann. 2018, 26, 710–712. [Google Scholar] [CrossRef]
- Agrawal, C.; Talwar, V.; Tayal, J.; Koyyala, V.B.; Goyal, P. Primary pulmonary melanoma: An unexpected diagnosis. Indian J. Pathol. Microbiol. 2018, 61, 636. [Google Scholar] [CrossRef]
- Azuma, Y.; Ono, H.; Kawabe, K.; Yanagimoto, R.; Suruda, T.; Minakata, Y. Primary pulmonary melanoma diagnosed by semi-rigid thoracoscopy. Thorac. Cancer 2018, 9, 1528–1529. [Google Scholar] [CrossRef]
- Shi, Y.; Bing, Z.; Xu, X.; Cui, Y. Primary pulmonary malignant melanoma: Case report and literature review. Thorac. Cancer 2018, 9, 1185–1189. [Google Scholar] [CrossRef]
- Peng, J.; Han, F.; Yang, T.; Sun, J.; Guan, W.; Guo, X. Primary malignant melanoma of the lung. Medicine 2017, 96, e8772. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.; Zarkavelis, G.; Andrianopoulou, A.; Papoudou-Bai, A.; Stefanou, D.; Boussios, S.; Pentheroudakis, G. Primary Pulmonary Malignant Melanoma: Report of an Important Entity and Literature Review. Case Rep. Oncol. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Froudarakis, M.; Da Cruz, V.; Forest, F.; Sauvage, M.; Vergnon, J.M.; Peoc’H, M. Endobronchial melanoma metastasis 40 years after the excision of the primary cutaneous tumor. Medicine 2017, 96, e7931. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kodama, K.; Maniwa, T.; Takeda, M.; Tanaka, Y.; Ozawa, K.; Isei, T. Primary malignant melanoma of the lung: A case report. Mol. Clin. Oncol. 2017, 7, 39–41. [Google Scholar] [CrossRef]
- Baniak, N.; Podberezin, M.; Kanthan, S.; Kanthan, R. Primary pulmonary/pleural melanoma in a 13-year-old presenting as pleural effusion. Pathol. Res. Pr. 2017, 213, 161–164. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, J.; Yang, Q.; Xiong, W.; Zhen, G.; Xu, Y.; Zhang, Z.; Zhang, H. Pulmonary melanoma and “crazy paving” patterns in chest images: A case report and literature review. BMC Cancer 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Kim, S.R.; Yoon, H.; Jin, G.Y.; Choe, Y.H.; Park, S.Y.; Lee, Y.C. Pulmonary malignant melanoma with distant metastasis assessed by positron emission tomography-computed tomography. Thorac. Cancer 2016, 7, 503–507. [Google Scholar] [CrossRef]
- Gupta, A.; Bhattacharya, D.; Jain, S.; Suri, J.C. Primary Malignant Melanoma of the Lung: Case Report and Literature Review. Indian J. Chest Dis. Allied Sci. 2016, 57, 181–184. [Google Scholar]
- Filippini, A.; Zorzi, F.; Bna’, C.; Arnaboldi, A.; Sabatini, T. Dark sputum: An atypical presentation of primary pulmonary malignant melanoma. Respir. Med. Case Rep. 2015, 15, 118–120. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamamoto, H.; Hashida, S.; Soh, J.; Sugimoto, S.; Toyooka, S.; Miyoshi, S. Primary pulmonary melanoma: A report of two cases. World J. Surg. Oncol. 2015, 13, 1–5. [Google Scholar] [CrossRef]
- Postrzech-Adamczyk, K.; Chabowski, M.; Głuszczyk-Ferenc, B.; Wodzińska, A.; Muszczyńska-Bernhard, B.; Szuba, A.; Janczak, D. Malignant melanoma of the lung: Case series. Pol. J. Card. Thor. Surg. 2015, 12, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-B.; Hwang, K.-E.; Jung, J.-W.; Oh, S.-J.; Park, M.-J.; Jeong, Y.-H.; Choi, K.-H.; Jeong, E.-T.; Kim, H.-R. Primary Pulmonary Malignant Melanoma: An Unexpected Tumor. Tuberc. Respir. Dis. 2015, 78, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Du, J. Primary malignant melanoma of left lower lobe of lung: A case report and review of the literature. Oncol. Lett. 2015, 10, 528–530. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahowald, M.K.; Aswad, B.; Okereke, I.C.; Ng, T. Long-Term Survival After Pneumonectomy for Primary Pulmonary Malignant Melanoma. Ann. Thorac. Surg. 2015, 99, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-H.; Liu, J.; Dong, H.; Tang, X.-J. Primary malignant melanoma of the lung: A case report. Int. J. Clin. Exp. Med. 2014, 7, 1757–1759. [Google Scholar]
- Kamaleshwaran, K.K.; Natarajan, S.; Parthiban, J.; Mehta, S.; Radhakrishnan, K.; Shinto, A.S. Rare case of extradural spinal metastasis from primary lung malignant melanoma detected with fluorine-18 fluorodeoxyglucose-positron emission tomography/computed tomography. Indian J. Nucl. Med. 2014, 29, 57–58. [Google Scholar] [CrossRef]
- Lazarou, I.; Purek, L.; Duc, C.; Licker, M.-J.; Spiliopoulos, A.; Tschopp, J.-M. Primary malignant achromic melanoma of the lung. Thorac. Cancer 2014, 5, 85–88. [Google Scholar] [CrossRef]
- Dos Santos, C.L.; Fernandes, L.R.; Meruje, M.; Barata, F. Primary pulmonary melanoma: The unexpected tumour. BMJ Case Rep. 2013, 2013, 200–706. [Google Scholar] [CrossRef]
- Gong, L.; Liu, X.-Y.; Zhang, W.-D.; Zhu, S.-J.; Yao, L.; Han, X.-J.; Lan, M.; Li, Y.; Zhang, W. Primary pulmonary malignant melanoma: A clinicopathologic study of two cases. Diagn. Pathol. 2012, 7, 123. [Google Scholar] [CrossRef]
- Ouarssani, A.; Atoini, F.; Reda, R.; Lhou, F.A.; Rguibi, M.I. Malignant melanoma of the lung: A case report. Pan Afr. Med. J. 2012, 11, 68. [Google Scholar]
- Zuckermann, B.; Papiashvilli, M.; Bar, I. Primary pulmonary malignant melanoma of right upper lobe of lung. IMAJ 2011, 13, 440–441. [Google Scholar]
- Pilozzi, E.; Cacchi, C.; Di Napoli, A.; Pini, B.; Duranti, E.; D’Andrilli, A.; Ruco, L. Primary malignant tumour of the lung with neuroendocrine and melanoma differentiation. Virchows. Archiv. 2011, 459, 239–243. [Google Scholar] [CrossRef]
- Seitelman, E.; Donenfeld, P.; Kay, K.; Takabe, K.; Andaz, S.; Fox, S. Successful treatment of primary pulmonary melanoma. J. Thorac. Dis. 2011, 3, 207–208. [Google Scholar]
- Neri, S.; Komatsu, T.; Kitamura, J.; Otsuka, K.; Katakami, N.; Takahashi, Y. Malignant Melanoma of the Lung: Report of Two Cases. Ann. Thorac. Cardiovasc. Surg. 2011, 17, 170–173. [Google Scholar] [CrossRef]
- Pan, X.-D.; Zhang, B.; Guo, L.-C.; Gu, D.-M.; Mao, Y.-Q.; Li, J.; Xie, Y.; Wang, L. Primary malignant melanoma of the lung in the elderly: Case report and literature review. Chin. Med. J. 2010, 123, 1815–1817. [Google Scholar]
- Maeda, R.; Isowa, N.; Onuma, H.; Miura, H.; Tokuyasu, H.; Kawasaki, Y. Primary malignant melanoma of the lung with rapid progression. Gen. Thorac. Cardiovasc. Surg. 2009, 57, 671–674. [Google Scholar] [CrossRef]
- Shikuma, K.; Omasa, M.; Yutaka, Y.; Okuda, M.; Taki, T. Treatment of Primary Melanoma of the Lung Monitored by 5-S-Cysteinyldopa Levels. Ann. Thorac. Surg. 2009, 87, 1264–1266. [Google Scholar] [CrossRef]
- Kotoulas, C.S.; Skagias, L.; Konstantinou, G.; Tsilalis, T.; Tsintiris, K.; Laoutidis, G.; Sambaziotis, D. Primary pulmonary melanoma diagnosis: The role of immunohistochemistry and immunocytochemistry. J. BUON 2007, 12, 543–545. [Google Scholar]
- Reddy, V.S.; Mykytenko, J.; Giltman, L.I.; Mansour, K.A. Primary Malignant Melanoma of the Lung: Review of Literature and Report of a Case. Am. Surg. 2007, 73, 287–289. [Google Scholar] [CrossRef]
- Kundranda, M.N.; Clark, C.T.; Chaudhry, A.A.; Chan, V.; Daw, H.A. Primary Malignant Melanoma of the Lung: A Case Report and Review of the Literature. Clin. Lung Cancer 2006, 7, 279–281. [Google Scholar] [CrossRef]
- Lie, C.-H.; Chao, T.-Y.; Chung, Y.-H.; Lin, M.-C. Primary pulmonary malignant melanoma presenting with haemoptysis. Melan. Res. 2005, 15, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Dountsis, A.; Zisis, C.; Karagianni, E.; Dahabreh, J. Primary Malignant Melanoma of the Lung: A Case Report. World J. Surg. Onc. 2003, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Iihara, K.; Yamaguchi, K.; Fujioka, Y.; Uno, S. Pigmented neuroendocrine tumor of the lung, showing neuromelanin. Pathol. Int. 2002, 52, 734–739. [Google Scholar] [CrossRef]
- Qureshi, R.; A Gazney, J.; Soorae, A.S. Rare pigmated tumor of the lung. J. Pak. Med. Assoc. 2002, 52, 183–185. [Google Scholar]
- Testini, M.; Trabucco, S.; Di Venere, B.; Piscitelli, D. Ileal intussusception due to intestinal metastases from primary malignant melanoma of the lung. Am. Surg. 2002, 68, 377–379. [Google Scholar]
- Özdemir, N.; Cangir, A.K.; Kutlay, H.; Yavuzer, Ş. Primary malignant melanoma of the lung in oculocutaneous albino patient. Eur. J. Card. Thorac. Surg. 2001, 20, 864–867. [Google Scholar] [CrossRef]
- Ost, D.E.; Joseph, C.; Sogoloff, H.; Menezes, G. Primary Pulmonary Melanoma: Case Report and Literature Review. Mayo. Clin. Proc. 1999, 74, 62–66. [Google Scholar] [CrossRef]
- Farrell, D.J.; Kashyap, A.P.; Ashcroft, T.; Morritt, G.N. Primary malignant melanoma of the bronchus. Thorax 1996, 51, 223–224. [Google Scholar] [CrossRef]
- Pasquini, E.; Rastelli, E.; Muretto, P.; Bonci, A.; Fattori, P.P.; Nicolini, M.; Ravaioli, A.; Pasini, P. Primary bronchial malignant melanoma. A case report. Pathologica 1994, 86, 546–548. [Google Scholar]
- Ueyama, T.; Tsuru, T.; Tsuneyoshi, M.; Sueishi, K.; Sibuya, T.; Fukuda, T. Primary Collision Neoplasm of Malignant Melanoma and Adenocarcinoma in the Lung. Pathol. Res. Pr. 1993, 189, 178–183. [Google Scholar] [CrossRef]
- Cohen, R.; Weaver, M.G.; Montenegro, H.D.; Abdul-Karim, F.W. Pulmonary blastoma with malignant melanoma component. Arch. Pathol. Lab. Med. 1990, 114, 1076–1078. [Google Scholar]
- Jennings, T.A.; Axiotis, C.A.; Kress, Y.; Carter, D. Primary Malignant Melanoma of the Lower Respiratory Tract: Report of a Case and Literature Review. Am. J. Clin. Pathol. 1990, 94, 649–655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Wilt, J.H.; Farmer, S.E.; Scolyer, R.A.; McCaughan, B.C.; Thompson, J.F. Isolated melanoma in the lung where there is no known primary site: Metastatic disease or primary lung tumour? Melan. Res. 2005, 15, 531–537. [Google Scholar] [CrossRef]
- Wilson, R.W.; Moran, C.A. Primary Melanoma of the Lung: A Clinicopathologic and Immunohistochemical Study of Eight Cases. Am. J. Surg. Pathol. 1997, 21, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.; Doulias, T.; Koutelidakis, I.; Papaziogas, B. Stage IV malignant melanoma of unknown primary site in a young man. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef]
- Rao, R.; Prasad, D.; Amareswar, A.; Vijaya, K.; Sujatha. Large congenital melanocytic nevus with metastatic melanoma with a probable primary in the lung. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 51. [Google Scholar] [CrossRef]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef]
- Colombino, M.; Sardinian Lung Cancer (SLC) Study Group; Paliogiannis, P.; Cossu, A.; Santeufemia, D.A.; Sini, M.C.; Casula, M.; Palomba, G.; Manca, A.; Pisano, M.; et al. EGFR, KRAS, BRAF, ALK, and cMET genetic alterations in 1440 Sardinian patients with lung adenocarcinoma. BMC Pulm. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo. Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Colombino, M.; Sini, M.; Lissia, A.; De Giorgi, V.; Stanganelli, I.; Ayala, F.; Massi, D.; Rubino, C.; Manca, A.; Paliogiannis, P.; et al. Discrepant alterations in main candidate genes among multiple primary melanomas. J. Transl. Med. 2014, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Sini, M.C.; Doneddu, V.; Paliogiannis, P.; Casula, M.; Colombino, M.; Manca, A.; Botti, G.; Ascierto, P.A.; Lissia, A.; Cossu, A.; et al. Genetic alterations in main candidate genes during melanoma progression. Oncotarget 2018, 9, 8531–8541. [Google Scholar] [CrossRef] [PubMed]
| Marker | Cases Tested, n | Positive Staining, n (%) | Negative Staining, n (%) |
|---|---|---|---|
| S-100 | 55 | 51 (92.7) | 4 (7.3) |
| HMB 45 | 53 | 50 (94.3) | 3 (5.7) |
| Cytokeratin | 38 | 0 (0) | 38 (100) |
| Melan-A | 21 | 18 (85.7) | 3 (14.3) |
| Chromorganin | 17 | 0 (0) | 17 (100) |
| CAM 5.2 | 13 | 1 (7.7) | 12 (92.3) |
| AE1/AE3 | 12 | 1 (8.3) | 11 (91.7) |
| Vimentin | 11 | 9 (81.8) | 2 (18.2) |
| CEA | 10 | 3 (30) | 7 (70) |
| NSE | 5 | 1 (20) | 4 (80) |
| Treatment | No (%) |
|---|---|
| Surgery (case with available data) | 72 |
| Total patients who underwent surgery | 51 (70.8) |
| Total surgical procedures | 56 |
| Lobectomy | 35 (62.5) |
| Wedge resection | 11 (19.6) |
| Pneumonectomy | 7 (12.5) |
| Broncoscopic resection | 2 (3.6) |
| Other/NA | 1 (1.8) |
| Chemotherapy (cases with available data) | 34 |
| Total patients treated | 20 (58.8) |
| Dacarbazine | 5 (25) |
| Dacarbazine, vincristine, nimustine | 3 (15) |
| Carboplatin | 1 (5) |
| Paclitaxel | 1 (5) |
| Vindesine, temozolomide, dacarbazine, carboplatin | 1 (5) |
| Other/NA | 1 (5) |
| Radiotherapy | 8 |
| Other therapies | 14 |
| Immunotherapy | 5 (35.7) |
| Interferon | 2 (14.3) |
| Interferon + immunotherapy | 2 (14.3) |
| Supportive care | 3 (21.4) |
| Targeted therapy + immunotherapy | 1 (7.1) |
| Other/NA | 1 (7.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paliogiannis, P.; Fara, A.M.; Pintus, G.; Abdel-Rahman, W.M.; Colombino, M.; Casula, M.; Palmieri, G.; Cossu, A. Primary Melanoma of the Lung: A Systematic Review. Medicina 2020, 56, 576. https://doi.org/10.3390/medicina56110576
Paliogiannis P, Fara AM, Pintus G, Abdel-Rahman WM, Colombino M, Casula M, Palmieri G, Cossu A. Primary Melanoma of the Lung: A Systematic Review. Medicina. 2020; 56(11):576. https://doi.org/10.3390/medicina56110576
Chicago/Turabian StylePaliogiannis, Panagiotis, Antonella M. Fara, Gianfranco Pintus, Wael M. Abdel-Rahman, Maria Colombino, Milena Casula, Giuseppe Palmieri, and Antonio Cossu. 2020. "Primary Melanoma of the Lung: A Systematic Review" Medicina 56, no. 11: 576. https://doi.org/10.3390/medicina56110576
APA StylePaliogiannis, P., Fara, A. M., Pintus, G., Abdel-Rahman, W. M., Colombino, M., Casula, M., Palmieri, G., & Cossu, A. (2020). Primary Melanoma of the Lung: A Systematic Review. Medicina, 56(11), 576. https://doi.org/10.3390/medicina56110576








