Risk of Complications and Survival of Patients Dialyzed with Permanent Catheters
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Methods
2.2. Ethical Statement
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lugon, J.R. Doenca renal cronica no Brasil; um problema de saude publico. J. Bras. Nefrol. 2009, 31, 2–5. [Google Scholar]
- National Kidney Fundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy, Vascular Access. Am. J. Kidney Dis. 2006, 47, S11–S145. [Google Scholar]
- Horan, T.C.; Gaynes, R.P.; Martone, W.J.; Jarvis, W.R.; Emori, T.G. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical would infections. Infect. Control. Hosp. Epidemiol. 1992, 13, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Załuska, W.; Klinger, M.; Kusztal, M.; Lichodziejewska-Niemierko, M.; Miłkowski, A.; Stompór, T.; Sak, J.; Domański, L.; Drożdż, M.; Aksamit, D.; et al. Recommendations of the Working Group of the Polish Society of Nephrology for the criteria of quality treatment in dialysis patients with end-stage renal disease. Nefrol. Dial. Pol. 2015, 19, 6–11. [Google Scholar]
- Depner, T.A.; Daugirdas, J.T.; Goldstein, S.; Ing, T.S.; Kumar, V.; Meyer, K.B.; Norris, K.; Balk, E.; Uhlig, K.; Fares, G.; et al. Clinical Practice Guidelines and Clinical Practice Recommendations 2006 Updates Hemodialysis Adequacy, Peritoneal Dialysis Adequacy, Vascular Access. Am. J. Kidney Dis. 2006, 48, S1–S322. [Google Scholar]
- Summary of Recommendation Statements Kidney International Supplements (2013, 3, 19-111). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Available online: http://www.kidney-international.org (accessed on 28 December 2012).
- Woo, K.; Lok, C.E. New Insights into Dialysis Vascular Access: What Is the Optimal Vascular Access Type and Timing of Access Creation in CKD and Dialysis Patients? Clin. J. Am. Soc. Nephrol. 2016, 11, 1487–1494. [Google Scholar] [CrossRef]
- Vats, H.S. Complications of Catheters: Tunneled and Nontunneled. Adv. Chronic Kidney Dis. 2012, 19, 188–194. [Google Scholar] [CrossRef]
- Białobrzeska, B. Jak dbać o dostęp naczyniowy do hemodializy (cz.2). Forum Nefrologiczne 2009, 2, 266–274. [Google Scholar]
- Labriola, L.; Pochet, J.-M. Any use for alternative lock solutions in the prevention of catheter-related blood stream infections? J. Vasc. Access 2017, 18, S34–S38. [Google Scholar] [CrossRef]
- Libeas-Lopez, J. New technology: Heparyn and atimicrobial-coated catheters. J. Vasc. Access 2015, 16, S48–S53. [Google Scholar] [CrossRef]
- Lai, N.M.; Chaiyakunapruk, N.; Lay, N.A.; O’Riordan, E.; Pau, W.S.; Saint, S. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst. Rev. 2013, 3, CD007878. [Google Scholar]
- Van Dijk, P.C.; Jager, K.J.; de Charro, F.; Collart, F.; Cornet, R.; Dekker, F.W.; Grönhagen-Riska, C.; Kramar, R.; Leivestad, T.; Simpson, K.; et al. Renal replacement therapy in Europe: The results of a collaborative effort by the ERA-EDTA registry and six national or regional registries. Nephrol. Dial. Transplant. 2001, 16, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Roca-Tey, R. Permanent arteriovenosus fistula or catheter dialysis for heart failure patients. J. Vasc. Access. 2016, 17, S23–S29. [Google Scholar] [CrossRef]
- Jain, D.; Haddad, D.B.; Goel, N. Choice of dialysis modality prior to kidney transplantation: Does it matter? World J. Nephrol. 2019, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leś, J.; Wańkowicz, Z. Cewniki długoterminowe do hemodializoterapii doświadczenia własne. Nefrol. Dial. Pol. 2013, 17, 19–23. [Google Scholar]
- Robinson, B.M.; Bieber, B.; Pisoni, R.L.; Port, F.K. Dialysis Outcames and Practice Patterns Study (DOOPS): Its Strengths, Limitations and Role in Informing Practices and Policies. Clin. J. Am. Soc. Nephrol. 2012, 7, 1897–1905. [Google Scholar] [CrossRef]
- Pawlaczyk, K. Prophylaxis and treatment of cardiovascular complications in patients with chronic renal failure. Przew Lek. 2009, 5, 47–50. [Google Scholar]
- Muntner, P.; He, J.; Astor, B.C.; Folsom, A.R.; Coresh, J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: Results from the atherosclerosis risk in communities study. J. Am. Soc. Nephrol. 2005, 16, 529–538. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Fried, L.F.; Cushman, M.; Manolio, T.A.; Peterson, D.; Stehman-Breen, C.; Bleyer, A.; Newman, A.; Siscovick, D.; Psaty, B. Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 2005, 293, 1737–1745. [Google Scholar] [CrossRef]
- Asif, A.; Bakr, M.M.; Levitt, M.; Vachharajani, T. Best Vascular Access in the Elderly: Time for Innovation? Blood Purif 2019, 47, 236–239. [Google Scholar] [CrossRef]
- Lee, T.; Mokrzycki, M.; Moist, L.; Maya, I.; Vazquez, M.; Lok, C.E.; North American Vascular Access Consortium. Standardized Definitions for Hemodialysis Vascular Access. Semin. Dial. 2011, 21, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Wołyniec, W.; Liberko, T.; Rutkowski, B. Cewniki tunelizowane w dializoterapii dobrodziejstwo czy zło konieczne? Forum Nefrologiczne 2015, 8, 205–213. [Google Scholar]
- Gellert, R.; Żelek, T.; Czystowski, M.; Daniewska, D. S-shaped-tunneled femoral catheter for permanent hemodialysis access. Postępy Nauk Medycznych 2010, 23, 604–607. [Google Scholar]
- Fry, A.C.; Stratton, J.; Farrington, K.; Mahna, K.; Selvakumar, S.; Thompson, H.; Warwicker, P. Factors affecting long-term survival of tunnelled haemodialysis catheters a prospective audit of 812 tunnelled catheters. Nephrol. Dial. Transplant. 2008, 23, 275–281. [Google Scholar] [CrossRef]
- Wejmer, M.C.; Vervloet, M.G.; ter Wee, P.M. Compared to tunnelled cuffed haemodialysis catheters, temporary untunnelled catheters are associated with more complications already within 2 weeks of use. Nephrol. Dial. Transplant. 2004, 19, 670–677. [Google Scholar] [CrossRef]
- Beathard, G.; Urbanes, A. Infection Associated with Tunneled Hemodialysis Catheters. Semin. Dial. 2008, 21, 528–538. [Google Scholar] [CrossRef]
- Lemaire, X.; Morena, M.; Leray-Moragués, H.; Henriet-Viprey, D.; Chenine, L.; Defez-Fougeron, C.; Canaud, B. Analysis of risk factors for catheter-related bacteremia in 2000 permanent dual catheters for hemodialysis. Blood Purif. 2009, 28, 21–28. [Google Scholar] [CrossRef]
- Engstrom, B.I.; Horvath, J.J.; Stewart, J.K.; Sydnor, R.H.; Miller, M.J.; Smith, T.P.; Kim, C.Y. Tunneled internal jugular hemodialysis catheters: Impact of laterality and tip position on catheter dysfunction and infection rates. J. Vasc. Interv. Radiol. 2013, 24, 1295–1302. [Google Scholar] [CrossRef]
- El Minschawy, O.; Abd El Aziz, T.; Abd El Ghani, H. Evaluation of vascular access complications in acute and chronic hemodialysis. J. Vasc. Access 2004, 5, 76–82. [Google Scholar] [CrossRef]
- Nelveg-Kristensen, K.E.; Laier, G.H.; Heaf, J.G. Risk of death after first-time blood stream infection in incident dialysis patients with specific consideration on vascular access and comorbidity. BMC Infect. Dis. 2018, 18, 688. [Google Scholar] [CrossRef]
- Brala, M.; Kamiński, R.; Trudnowski, S.; Grzybiak, M.; Kozłowski, D.; Raczak, G. Long-term complications after implantation of catheters for hemodialysis. Geriatrics 2014, 8, 1–5. [Google Scholar]
- Polkinghorne, K.R.; McDonald, S.P.; Atkins, R.C.; Kerr, P.G. Vascular access and all-cause mortality: A propensity score analysis. J. Am. Soc. Nephrol. 2004, 15, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Weyde, W.; Krajewska, M.; Klinger, M. Dostęp naczyniowy do hemodializy. Forum Nefrologiczne 2008, 3, 119–126. [Google Scholar]
- Quarello, F.; Forneris, G.; Borca, M.; Pozzato, M. Do central venous catheters have advantages over arteriovenous fistulas or grafts? J. Nephrol. 2006, 19, 265. [Google Scholar] [PubMed]
- Hamadneh, S.A.; Nueirat, S.A.; Qadoomi, J.; Shurrab, M.; Qunibi, W.Y.; Hamdan, Z. Vascular access aortality and hospitalisation among hemodialysis patients in Palestine. Saudi J. Kidney Dis. Transpl. 2018, 29, 120–126. [Google Scholar] [PubMed]
- Stenvinkel, P.; Carrero, J.J.; Axelsson, J.; Linholm, B.; Heimburger, O.; Massy, Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin. J. Am. Soc. Nephrol. 2008, 3, 505–521. [Google Scholar] [CrossRef]
- Wanner, C.; Zimmermann, J.; Schwedler, R.S.; Metzger, T. Inflammation and cardiovascular risk in dialysis patients. Kidney Int. Suppl. 2002, 80, 99–102. [Google Scholar] [CrossRef]
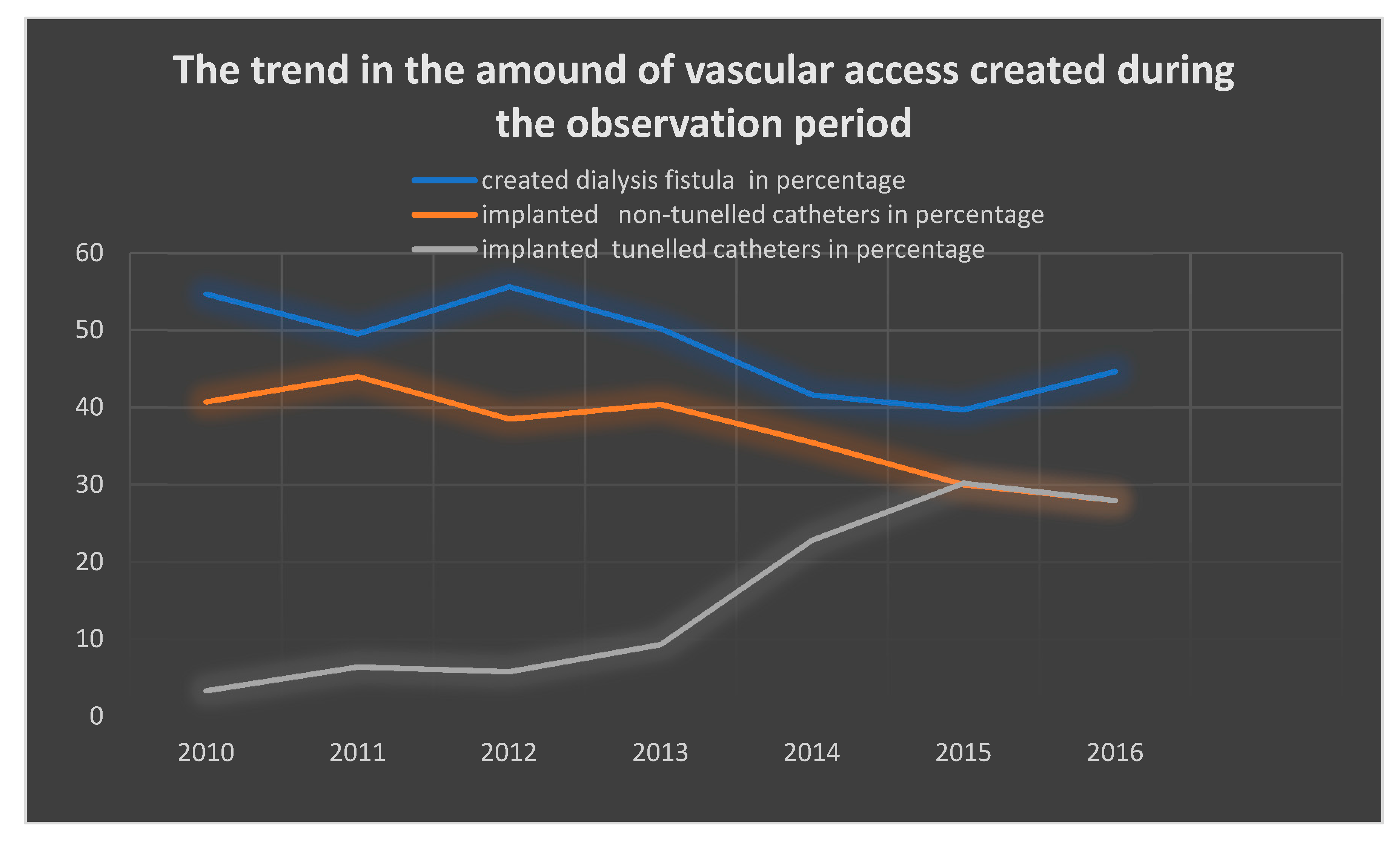

| All Patients | Women | Men | p-Value | |
|---|---|---|---|---|
| Number of patients (n, %) | 398 (100%) | 204 (51.25%) | 194 (48.75%) | NS |
| Age (years, SD) | 68.73 ± 13.26 | 69.50 ± 13.22 | 67.89 ± 13.28 | NS |
| The number of catheters inserted after dysfunction of previously formed dialysis shunt (n, %) | 129 (32.41) | 70 (34.31) | 59 (30.31) | NS |
| The number of patients with one catheter (n, %) | 322 (80.90%) | 160 (78.43%) | 162 (83.51%) | NS |
| The number of patients requiring one catheter replacement (n, %) | 58 (14.57%) | 37 (18.14%) | 21 (10.82%) | <0.1 |
| The number of patients requiring two or more catheter replacements (n, %) | 18 (4.52%) | 7 (3.33%) | 11 (5.67%) | NS |
| Time from first implantation of the catheter to the end of the study (days, SD) | 505.2 ± 428.3 | 508.9 ± 424.8 | 501.18 ± 433.0 | NS |
| The mean time of catheter functioning (days, SD) | 435.7 ± 398.2 | 439.7 ± 404.6 | 431.54 ± 392.4 | NS |
| The number of catheters replaced due to infection (n, %) | 10 (2.51) | 6 (2.94) | 4 (2.06) | NS |
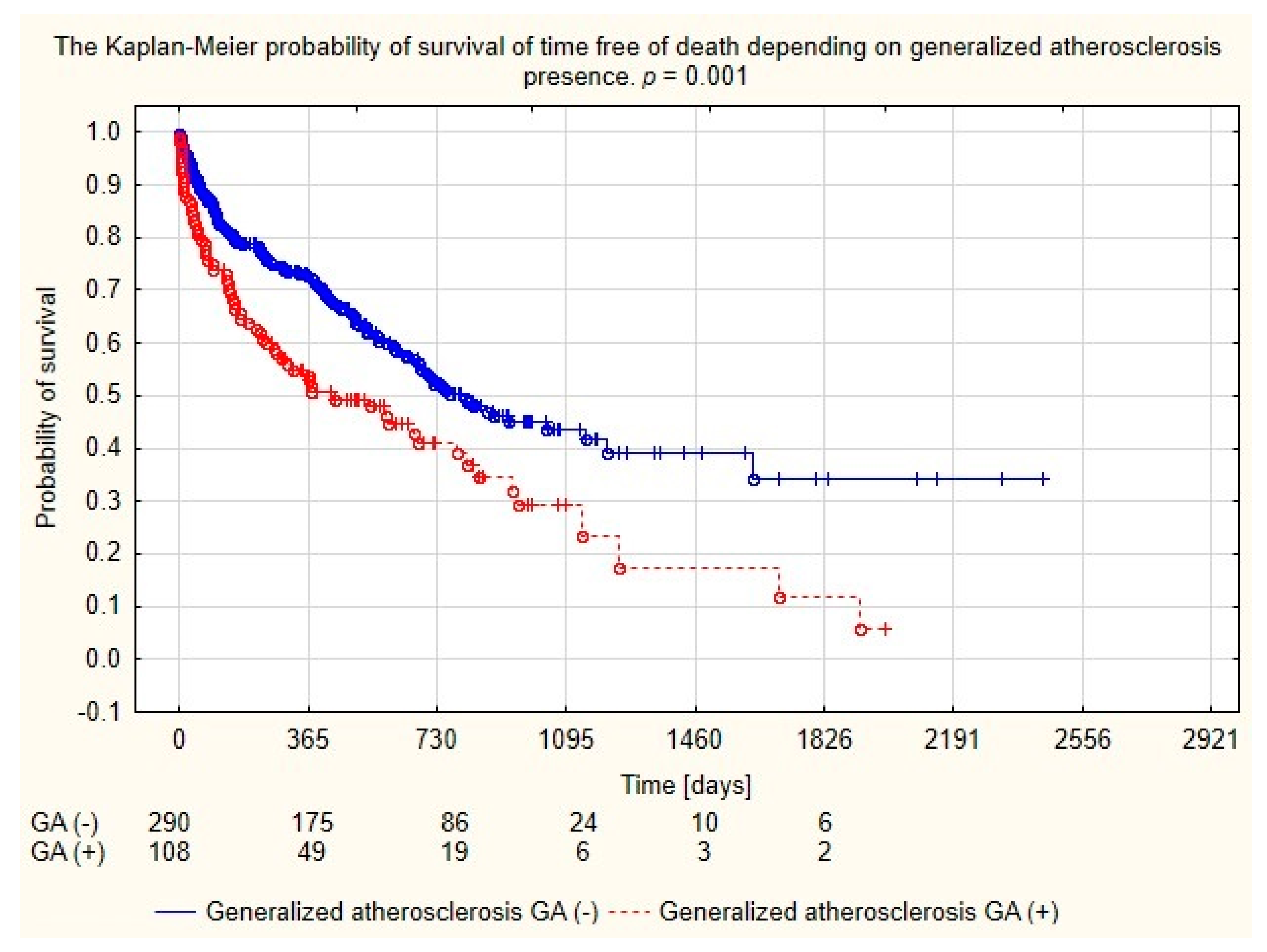
| Generalized atherosclerosis (n, %) 1 | 108 (27.14%) | 51 (25.00%) | 57 (23.98%) | NS |
| Thrombotic catheter complications (n, %) | 66 (16.58%) | 38 (18.63%) | 28 (14.43%) | NS |
| Diabetes (n, %) | 73 (18.34%) | 32 (15.69%) | 41 (21.13%) | NS |
| Coronary artery disease (n, %) | 44 (11.06%) | 23 (11.27%) | 21 (10.82%) | NS |
| Hypertension (n, %) | 109 (27.39%) | 53 (25.98%) | 56 (28.87%) | NS |
| Heart failure III-IV acc. to NYHA (n, %) | 78 (19.60%) | 35 (17.16%) | 43 (22.16%) | NS |
| Stroke in the history (n, %) | 4 (1.01%) | 3 (1.47%) | 1 (0.52%) | NS |
| Chronic atrial fibrillation (n, %) | 12 (3.02%) | 7 (3.43%) | 5 (2.58%) | NS |
| Neoplastic disease (n, %) | 78 (19.60%) | 35 (17.16%) | 43 (22.16%) | NS |
| Other diseases (n, %) 2 | 87 (21.86%) | 41 (20.60%) | 46 (23.12%) | NS |
| The number of all local and generalized infectious complications (n, %) | 92 (23.12%) | 36 (17.65%) | 56 (28.87%) | p < 0.05 |
| The number of patients with local and generalized infectious complications (n, %) | 65 (16.33%) | 29 (14.22%) | 36 (18.56%) | NS |
| Decease (n, %) | 199 (50.00%) | 103 (50.49%) | 96 (49.48%) | NS |
| Patients with a Permanent Catheter Implanted after Dysfunction of a Previously Created Dialysis Fistula | Patients with a Permanent Catheter Implanted as the First Access | p-Value | |
|---|---|---|---|
| Number of patients (n, %) | 129 (32.41) | 269 (67.59) | |
| Age (years, SD) | 66.202 ± 11.799 | 69.944 ± 13.765 | <0.001 |
| Female (n, %) | 70 (54.26) | 134 (49.81) | NS |
| Time from the first catheter implantation to the end of the follow-up (days, SD) | 565.248 ± 409.284 | 476.342 ± 434.248 | <0.001 |
| Mean time of catheter functioning (days, SD) | 449.721 ± 353.274 | 429.037 ± 418.505 | NS |
| The number of patients with one catheter (n, %) | 92 (71.32) | 230 (85.50) | 0.001 |
| The number of patients requiring one catheter replacement (n, %) | 26 (20.16) | 32 (11.90) | 0.042 |
| The number of patients requiring two or more catheter replacements (n, %) | 11 (8.53) | 7 (2.60) | 0,016 |
| Thrombotic catheter complications (n, %) | 30 (23.26) | 36 (13.38) | 0.020 |
| Diabetes (n, %) | 19 (14.73) | 54 (20.07) | 0.001 |
| Generalized atherosclerosis (n, %) | 26 (20,16) | 82 (30,48) | 0,041 |
| Coronary artery disease (n, %) | 12 (9.30) | 32 (11.90) | NS |
| Hypertension (n, %) | 30 (23.26) | 79 (29.37) | NS |
| Heart failure III-IV acc. to NYHA (n, %) | 27 (20.93) | 51 (18.96) | NS |
| Stroke in the history (n, %) | 2 (1.55) | 2 (0.74) | NS |
| Atrial fibrillation (n, %) | 3 (2.33) | 9 (3.35) | NS |
| Neoplastic disease (n, %) | 17 (13.18) | 61 (22.68) | 0.036 |
| The number of all local and generalized infectious complications (n, %) | 34 (26.36) | 58 (21.56) | NS |
| The number of patients with local and generalized infectious complications (n, %) | 26 (20.16) | 39 (14.49) | NS |
| Decease (n, %) | 56 (43.41) | 143 (53.16) | NS (0.087) |
| Univariate Logistic Regression | |||
| Parameter | HR | 95% CI | p-Value |
| Patient’s age | 1.021 | 1.005–1038 | 0.009 |
| A disease of atherosclerotic etiology (yes/no) 1 | 1.737 | 1.049–2.875 | 0.031 |
| Diabetes | 1.454 | 0.820–2.578 | NS |
| Neoplastic disease | 1.932 | 1.075–3.473 | 0.027 |
| Other diseases 2 | 1.672 | 0.969–2.885 | NS (0.064) |
| Heart failure III-IV according to NYHA classification | 0.884 | 0.523–1.493 | NS |
| Multivariate Logistic Regression | |||
| Patient’s age | 1.017 | 0.999–1.036 | NS (0.060) |
| A disease of atherosclerotic etiology 1 (yes/no) | 1.278 | 0.722–2.263 | NS |
| Neoplastic disease | 1.728 | 0.950–3.143 | NS (0.073) |
| Other diseases 2 | 1.531 | 0.868–2.700 | NS |
| Study Group | Decease | Living Patients | p-Value | |
|---|---|---|---|---|
| The number of patients (n) | 398 | 199 | 199 | |
| Sex (women) (n, %) | 204 (51.26) | 103 (51.76) | 101 (50.75) | NS |
| Patient’s age (years ± SD) | 68.88 ± 13.29 | 72.12 ± 11.65 | 67.18 ± 13.64 | <0.001 |
| Presence of an earlier dialysis fistula (n, %) | 129 (32.41%) | 56 (28.15%) | 73 (36.68%) | NS (0.086) |
| The number of patients with one catheter (n, %) | 322 (80.90%) | 163 (81.91%) | 159 (79.90%) | NS |
| The number of patients requiring one catheter replacement (n, %) | 58 (14.57%) | 27 (13.57%) | 31 (15.58%) | NS |
| The number of patients with two or more catheter replacements (n, %) | 18 (4.52%) | 9 (4.52%) | 9 (4.52%) | NS |
| The number of catheters replaced due to infection (n, %) | 10 (2.51) | 5 (2.51%) | 5 (2.51%) | NS |
| The number of catheters replaced due to dysfunction (n, %) | 66 (16.58) | 33 (16.58%) | 33 (16.58%) | NS |
| Time from the first catheter implantation to the end of the study (days, SD) | 505.2 ± 428.3 | 317.0 ± 342.7 | 693.3 ± 423.2 | <0.001 |
| Mean time of catheter functioning (days, SD) | 435.7 ± 398.2 | 256.6 ± 270.8 | 614.9 ± 424.4 | <0.001 |
| Thrombotic catheter complications (n, %) | 66 (16.58%) | 33 (16.58%) | 33 (16.58%) | NS |
| Infectious complications (n,%) | 65 (16.33%) | 35 (17.59%) | 30 (15.08%) | NS |
| Diabetes mellitus (n, %) | 58 (14.57%) | 28 (14.07%) | 30 (15.08%) | NS |
| Generalized atherosclerosis (n, %) | 108 (27.14%) | 67 (33.67%) | 41 (20.60%) | <0.01 |
| Coronary artery disease (n, %) | 44 (11.06%) | 22 (11.06%) | 22 (11.06%) | NS |
| Hypertension (n, %) | 109 (27.39%) | 56 (28.14%) | 53 (26.63%) | NS |
| Atrial fibrillation (n, %) | 12 (3.02%) | 6 (3.02%) | 6 (3.02%) | NS |
| Heart failure II/IV according to NYHA classification (n, %) | 78 (19.60%) | 43 (21.61%) | 35 (17.59%) | NS |
| Stroke in the history (n, %) | 4 (1.01%) | 3 (1.51%) | 1 (0.50%) | NS |
| Neoplastic disease (n, %) | 78 (19.60%) | 36 (18.09%) | 42 (21.11%) | NS |
| Decease Risk Cox-Univariate | HR | 95% CI | p-Value |
| Patient’s age | 1.035 | 1.023–1.049 | 0.000 |
| Generalized atherosclerosis | 1.662 | 1.238–2.232 | 0.000 |
| Decease Risk Cox-Bivariate | HR | 95% CI | p-Value |
| Patient’s age | 1.034 | 1.020–1.050 | 0.000 |
| Generalized atherosclerosis | 1.062 | 0.752–1.500 | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szarnecka-Sojda, A.; Jacheć, W.; Polewczyk, M.; Łętek, A.; Miszczuk, J.; Polewczyk, A. Risk of Complications and Survival of Patients Dialyzed with Permanent Catheters. Medicina 2020, 56, 2. https://doi.org/10.3390/medicina56010002
Szarnecka-Sojda A, Jacheć W, Polewczyk M, Łętek A, Miszczuk J, Polewczyk A. Risk of Complications and Survival of Patients Dialyzed with Permanent Catheters. Medicina. 2020; 56(1):2. https://doi.org/10.3390/medicina56010002
Chicago/Turabian StyleSzarnecka-Sojda, Anna, Wojciech Jacheć, Maciej Polewczyk, Agnieszka Łętek, Jarosław Miszczuk, and Anna Polewczyk. 2020. "Risk of Complications and Survival of Patients Dialyzed with Permanent Catheters" Medicina 56, no. 1: 2. https://doi.org/10.3390/medicina56010002
APA StyleSzarnecka-Sojda, A., Jacheć, W., Polewczyk, M., Łętek, A., Miszczuk, J., & Polewczyk, A. (2020). Risk of Complications and Survival of Patients Dialyzed with Permanent Catheters. Medicina, 56(1), 2. https://doi.org/10.3390/medicina56010002





